Download PDF
Ophthalmic pathology is undergoing a transformation. The field “has progressed from looking at glass slides to a broader specialty incorporating molecular biology and gene testing, giving us novel insight that is leading to the ability to provide more accurate diagnoses, prognoses, and, ultimately, targeted therapy,” said Deepak P. Edward, MD, at the University of Illinois in Chicago.
Hans E. Grossniklaus, MD, MBA, predicted this move toward molecular and digital pathology in the September 2002 issue of EyeNet in an article titled “Pathology at the Crossroads”—at a time when slides were first being transferred to computers and six months before the Human Genome Project was completed in April 2003.
“And 17 years later, from glaucoma to age-related macular degeneration to retinoblastoma, advances in molecular pathology are leading to breakthroughs in disease management,” said Dr. Grossniklaus, at Emory University in Atlanta.
The evolution of ocular pathology has also affected collaborations among subspecialists (see “The Oncology-Pathology Link”) and even nomenclature (see “What’s in a Name?”).
“New tools and molecular breakthroughs are allowing us to define the pathogenesis of disease, thus moving us closer” to precision medicine, Dr. Edward pointed out. “And the field is changing all the time.” The most vivid manifestations of these advances are in the clinic—notably in retinoblastoma and uveal melanoma. And the underpinnings for these advances involve the interplay between research, imaging and technology, and clinical care.
 |
|
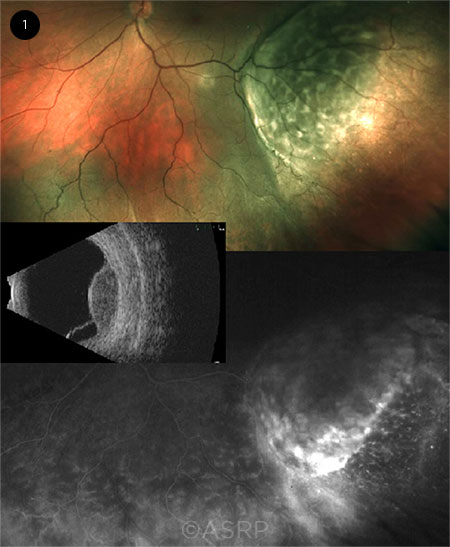
GENE PROFILING. (1) The GEP assay allows clinicians to fine-tune their diagnosis of entities such as the choroidal mass shown here. This image was originally published in the ASRS Retina Image Bank. Sarah Oelrich. Choroidal Mass. Retina Image Bank. 2018; Image Number 28591. © The American Society of Retina Specialists.
|
Clinical Advance: Retinoblastoma
In the early 2000s, Patricia Chévez-Barrios, MD, was at the forefront of the emergence of molecular pathology, focusing on retinoblastoma (RB) and its unique features in an effort to describe the tumor’s characteristics and patterns of metastases. While most cases could be cured with enucleation, ophthalmologists attempted to salvage the eye using chemotherapy and radiotherapy to shrink the tumors so they could be ablated with lasers and/or cryotherapy.
“We found, however, that when vitreous seeds (small pieces of avascular tumor floating in the vitreous) were present, the therapies were usually unsuccessful,” said Dr. Chévez-Barrios, at Houston Methodist in Texas. This insight led to research on the feasibility of adenovirus-media gene therapy as a treatment for the tumor seeds in RB patients. The treatment involved dose escalation of adenoviral vector containing a herpes simplex thymidine kinase gene (AdV-TK), followed by a systemic administration of ganciclovir—and the researchers found that it can safely and effectively be administered to children with RB and may be a viable and safe approach to RB tumor seeds.1
“We were the first in the world to use intraocular gene therapy, and 15 years later we continue to focus on RB, looking at microscopic findings and their impact on the clinical picture,” Dr. Chévez-Barrios said. For instance, she said, in a study published in 2017, “we looked at the vitreous seeds in retinoblastoma eyes that were enucleated and noticed that their individual characteristic correlated with the way they respond to chemotherapy.”2
Ophthalmic pathology continues to lead advances in RB, Dr. Chévez-Barrios said, citing recent breakthroughs by Jesse L. Berry, MD, in the area of liquid biopsy research using the aqueous humor of eyes with RB, along with a potential biomarker for the disease in aqueous humor (see “Liquid Biopsy in Retinoblastoma Research” in the April 2019 EyeNet).
 |
|
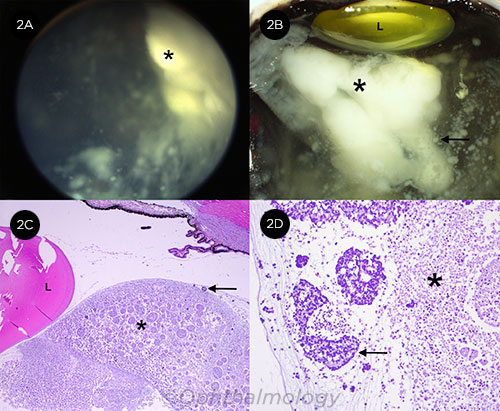
RETINOBLASTOMA. (2A) A type 3 vitreous seed (*), with types 1 and 2 seeds visible. (2B) A large cloud-type seed (*) behind the lens (L), with a sphere-type vitreous seed opening into the white portion of the cloud seed (arrow). (2C) Histologic correlate of the cloud seed (*) shows massive necrosis of the tumor behind the lens (L) and the sphere-type seed opening into the cloud (arrow). (2D) Higher magnification of the seed (*), with the opened sphere releasing necrotic material into the cloud (arrow).
|
Clinical Advance: Uveal Melanoma
The statistics on uveal melanoma are daunting. It is the most common primary intraocular malignancy in adults, and 97% to 98% of patients present with no evidence of metastatic disease at the time of diagnosis.3 While the success rate for local treatment exceeds 90%, half of all patients will ultimately develop metastatic disease, with a high percentage of cases metastasizing to the liver, followed by the lung and bones.
Predicting metastatic risk. “For many, many years we treated primary melanoma as an ocular tumor alone, even though the possibility of metastases always loomed,” said Zélia Maria Corrêa, MD, PhD, at the Wilmer Eye Institute in Baltimore.
This changed following publication of a study by the Collaborative Ocular Oncology Group (COOG), noted Dr. Corrêa, a member of COOG. Results of this study demonstrated the viability of a 15-gene expression profiling (GEP) assay that assigned primary posterior uveal melanomas to two prognostic subgroups in terms of subsequent metastatic risk.4 The GEP assay successfully classified 446 of 459 cases (97.2%). Specifically, patients with Class 1 disease had low metastatic risk, and those with Class 2 had high metastatic risk.
This has allowed clinicians such as Dr. Chévez-Barrios to conduct rapid onsite real-time assessment—evaluating a drop of fresh tumor aspirate from the needle biopsy drawn in the OR. “We immediately know if the surgeon is sampling the tumor or normal tissue and which type of tumor the patient has,” she said. “It is extremely useful information to have because this confirms that the prognostic results that the ocular oncologist receives are coming from the tumor—and this [information] can confidently be shared with the patient and medical team.”
Although the GEP tests are excellent in terms of accuracy, “We want to learn more” with regard to prognosis and survival, Dr. Corrêa said. She explained that a deeper investigation of the patients in the clinical studies produced outliers—that is, patients who were thought to be high risk were actually low risk and vice versa.
COOG “is now looking at genomic, analytic, and sequencing technologies to discover major drive mutations for possible use in targeted therapy,” Dr. Corrêa said. She concluded, “Ultimately, we want to tailor adjuvant treatments and create immunotherapy strategies that can actually beat this disease.”
 |
|
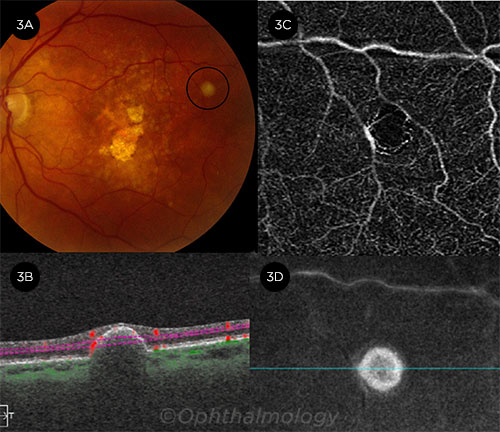
ASTROCYTIC MASS. (3A) A chronic asymptomatic pearl-white lesion in the temporal macula, consistent with solitary circumscribed retinal astrocytic proliferation. (3B) B-scan shows a mass arising from the outer retina or retinal pigment epithelium with inward retinal compression. (3C) OCTA of the deep capillary plexus shows no intrinsic vascularity. (3D) The en face image of the midretina.
|
From Bench to Bedside
Advances in ophthalmic pathology also are providing more opportunities for translational research. Dr. Grossniklaus has both a clinical practice for ocular oncology patients and an ophthalmic pathology lab. “I can see firsthand how these [research] advances improve patient care and outcomes,” he said.
Uveal melanoma. In one of his projects, Dr. Grossniklaus contributed to the bench research involving AU-011 (Aura Biosciences), a novel recombinant papillomavirus-like particle drug conjugate with potential to treat primary uveal melanoma.5
“We studied the anticancer properties of AU-011 in vitro using a panel of human cancer cell lines and in vivo using murine subcutaneous and rabbit orthotopic xenograft models of uveal melanoma,” Dr. Grossniklaus said. “Based on these findings, the company has launched a clinical trial.”
This line of inquiry “really is taking ophthalmic pathology from bench to bedside,” he added. “I was able to treat the rabbits as an experimental oncologist, study the treatment as a pathologist, and now hopefully will be treating patients.”
RAP secondary to AMD. The “bedside” aspect of ophthalmic pathology extends to contributing insight into the disease process. David J. Wilson, MD, and his colleagues recently explored retinal angiomatous proliferation (RAP) secondary to age-related macular degeneration (AMD) treated with serial ranibizumab injections and its pathologic correlation to imaging findings.6
“We looked at both the postmortem histopathologic features of a 93-year-old man’s eye and findings on spectral-domain optical coherence tomography (SD-OCT) seven weeks before his death,” said Dr. Wilson, at Oregon Health & Science University in Portland. The histopathologic findings corresponded to an area of hyperreflectivity on the SD-OCT, thus substantiating the reported tomographic appearance of RAP lesions.
“With our ability to correlate the findings of patients in the clinic with molecular-level knowledge, we can make the management of eye disease more precise,” Dr. Wilson said.
 |
|
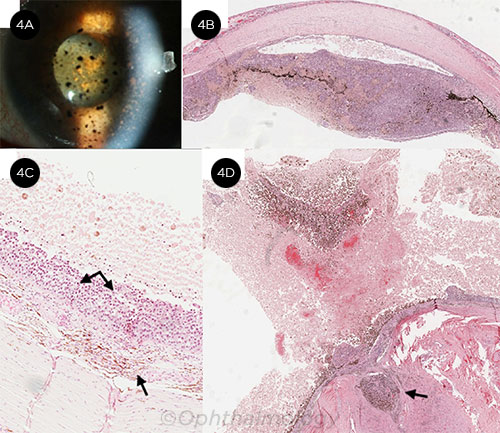
METASTATIC MELANOMA. This patient presented with aggressive cutaneous BRAF wild-type melanoma to the left eye, brain, and soft tissues. (4A) Free-floating melanocytic cells in the anterior chamber. (4B) Melanoma replaced iris tissue and (4C) the retina (double arrows) while the choroid (single arrow) and Bruch membrane were spared. (4D) Local extension to the optic nerve (arrow) was noted.
|
Imaging and Digital Applications
As with other areas in ophthalmology, ocular pathology is riding the digital and imaging wave. “We are replacing the [slide scanning] machines that cost tens of thousands of dollars with smartphone technology and adapters,” Dr. Chévez-Barrios noted.
Telepathology. Digital pathology has implications in terms of both teaching and patient care. Dr. Edward spent most of this past spring in Riyadh, Saudi Arabia, pursuing his research. He was able to review findings with his fellows and residents back in Chicago by scanning his ocular slides and sharing them via the internet. “This really is extraordinary, to be able to share pathology findings in real time half a world away,” he said.
Previously, if he was overseas and needed to do a consultation for a patient in the United States, the patient’s ophthalmologist would have to ship the slides and navigate customs—and it would still take a month to receive them via FedEx. Digital pathology now allows Dr. Edward to immediately diagnose the patient, thus eliminating shipping hassles and time limitations.
Digital pathology also allows Dr. Chévez-Barrios to work with physicians in developing countries that do not have any ocular pathologists. “For example, I am working with an informatics fellow in pathology to hold tumor board exams via virtual telepathology,” she said.
Virtual microscopy. Through a synthesis of microscopy and digital technologies, it is now possible to post and transmit microscope images on computer networks—and the image resolutions are approaching those visible under the optical microscope.7
A virtual microscopy work group, the Ophthalmic Pathology Collaborative and Educational Resource, is building a large database of high-quality histopathologic scanned specimens that will be made available to interested parties worldwide. The group includes ophthalmologists, ophthalmology residents, and medical students from institutions across the country.
OCT applications. One of Dr. Wilson’s projects involves using knowledge gained from eye pathology to interpret in vivo images obtained with OCT angiography (OCTA).
From histology findings, Dr. Wilson had previously identified four human retinal vascular plexuses. However, OCTA can only discriminate vascular patterns on the more superficial plexuses because flow projection artifact—which is caused by the flickering shadow from superficial blood flow being projected onto deeper tissue layers—limits the device’s ability to obtain a clean image of the deeper vascular plexuses.
To address this challenge, Dr. Wilson and his colleagues created a novel algorithm called “projected-resolved” OCTA. The algorithm improves depth resolution by removing projection artifact while retaining the flow signal from blood vessels in deeper levels,8 thus helping the researchers identify four distinct vascular plexuses in the retina. Armed with this information, the researchers proposed creating an improved system of nomenclature and segmentation boundaries for use with OCTA.
“Being able to correlate the images we take of the eye with the progressively detailed analysis in the laboratory enhances our ability to manage disease,” Dr. Wilson pointed out.
Preclinical evaluation. In another study, conducted in rhesus macaques, Dr. Wilson and his colleagues used a combination of imaging techniques and immunohistochemistry to evaluate the intraocular immune response following transplantation of allogeneic retinal pigment epithelial (RPE) cells into the subretinal space.9
The research team discovered that the engraftment of the allogeneic RPE cells failed followingthe transplantation, a finding likely due to rejection by the immune system. This led to the recommendation that RPE cell transplantation requires autologous cell sources and/or adequate immune suppression.
The Oncology-Pathology Link
Historically, ophthalmic pathology has played a significant role in advancing ophthalmology, as it has “added value to the practice of ophthalmology by structurally defining and understanding ophthalmic disease processes, which in turn led to medical and surgical treatments.”1
But not that long ago, ophthalmic pathology appeared to have hit a plateau, with some observers questioning the field’s viability. One step that reinvigorated the subspecialty: joining forces with ocular oncology.
Indeed, in 2012, the American Association of Ophthalmic Pathologists became the American Association of Ophthalmic Oncologists and Pathologists.
“Many of the advances in ophthalmic oncology have been linked to pathology,” said Bita Esmaeli, MD, at the University of Texas MD Anderson Cancer Center in Houston. In her orbital oncology and oncologic ophthalmic plastic surgery practice, she said, “I see a wide variety of cancers and rely on my pathology colleagues to provide a correct diagnosis as well as to guide us in creating tumor-free margins. Our pathologists are a huge part of my research collaboration and, from a practical standpoint, are greatly involved in day-to-day patient care.”
This collaboration has resulted in a number of peer-reviewed publications. For instance, Dr. Esmaeli and her pathology colleagues published a case series report suggesting that anti–PD-1 (inhibitors of programmed cell death 1 protein) therapy can be used to treat metastatic conjunctival melanoma.2 In this study, four patients with metastatic conjunctival melanoma treated with nivolumab had a complete response to treatment with no evidence of disease up to 36 months after finishing treatment. Given the findings, the researchers called for future studies to determine whether immune checkpoint inhibitors could obviate the need for orbital exenteration in certain patients with locally advanced disease.
“Personalized targeted therapy for ophthalmic cancer patients is truly an advance, one that could not have been accomplished without the contributions of pathologists,” Dr. Esmaeli said. “We have a strong partnership, and this collaboration is crucial to advancing care in our patients.”
___________________________
1 Grossniklaus H. Ophthalmology. 2015;122:1539-1542.
2 Sagiv O et al. JAMA Ophthalmol. 2018;136(11):1236-1241.
|
Looking Ahead
As molecular biology and digital technology continue on into uncharted territory, what’s next for ophthalmic pathology?
Thinking outside the box. Two recent research projects illustrate the value of looking beyond ophthalmic pathology—in this case, dermatology and cardiology—in an effort to develop targeted, personalized therapies for eye cancers.
Dermatology. “One of our studies involves looking at the role of immunohistochemical studies to help us distinguish benign from malignant conjunctival lesions,” said Tatyana Milman, MD, at Wills Eye Hospital in Philadelphia. “We are modeling our study on the most recent data from dermatopathology literature, which delineates the approach to using immunohistochemical stains in improving diagnostic accuracy for challenging cutaneous melanocytic lesions and for prognostication of skin melanomas.”
Cardiology. The second project focuses on conjunctival myxomas, which are rare tumors of the ocular surface. “There is some dispute regarding the precise nature of these lesions, with some experts favoring neoplastic processes, while others suggest that these lesions are reactive phenomena,” Dr. Milman said.
Conjunctival myxomas can occur in association with Carney complex. In this cancer predisposition syndrome, patients have various benign and malignant tumors, including myxomas, in several organs—most frequently the heart, Dr. Milman said. She explained, “We are applying the most recent methods from molecular genetic studies on cardiac myxomas to the conjunctival myxoid tumors to see if we can define which conjunctival myxoid lesions are truly neoplastic and which merit potential investigation for a genetic syndrome.”
Artificial intelligence. AI is expected to play a significant role in ophthalmic pathology, given its ability to analyze images in both the path lab and the clinic. “High-level computing can make correlations that we aren’t able to figure out on our own,” noted Dr. Wilson. “AI holds great promise in terms of advancing what we understand about disease and helping us better manage patients.”
Dr. Chévez-Barrios added, “AI will not replace the pathologist, but it can provide insights about exact molecular mutations as well as identify patterns in diseases that we may not be able to detect without AI.”
What’s in a Name?
Recent discoveries in ophthalmic pathology may lead to changes in the nomenclature.
Based on research findings, some experts have argued that the lesion known as retinal vasoproliferative tumor should now be called retinal reactive astrocytic tumor.1,2
This benign tumor has glial cell and vascular components well-characterized through histopathology. Yet further exploration through gene expression profiling confirmed that while some genes are overexpressed in the condition, others are underexpressed. Specifically, the most significantly upregulated genes were expressed in both astrocytes and reactive astrocytes, thus the proposed name change.
___________________________
1 Poole Perry LJ. Am J Ophthalmol. 2013;155(3):593-608.
2 Shehri M et al. JAMA Ophthalmol. 2014;132(6):773-775.
|
The Bottom Line
As ophthalmology moves toward increasingly sophisticated and personalized treatments, “ophthalmologists should take more advantage of the ophthalmic pathologist’s expertise,” Dr. Corrêa said. She added, “Many times, we miss the opportunity to do an early intervention because we rely on the appearance and not the histopathology or molecular biology. Pathologists are, and will remain, an important resource.”
___________________________
1 Chévez-Barrios et al. J Clin Oncol. 2005;23(31):7927-7935.
2 Amram AL et al. Ophthalmology. 2017;124(10):1540-1547.
3 Francis JH et al. The Melanoma Letter. Fall 2016;34(3). www.skincancer.org/publications/the-melanoma-letter/fall-2016-vol-34-no-3. Accessed June 18, 2019.
4 Onken MD et al. Ophthalmology. 2012;119(8):1596-1603.
5 Kines RC et al. Mol Cancer Ther. 2018;17(2):565-574.
6 Skalet AH et al. Retina. 2017;37(8):1620-1624.
7 www.loyolamedicine.org/gme/ophthalmology-residency/opvm. Accessed June 18, 2019.
8 Campbell JP et al. Sci. Rep. 2017;7:42201.
9 McGill TJ et al. Invest Ophthalmol Vis Sci. 2018;59(3):1374-1383.
Meet the Experts
Patricia Chévez-Barrios, MD Professor of pathology and genomic medicine and ophthalmology at the Institute for Academic Medicine at Houston Methodist and Weill Cornell Medical College in New York, N.Y. She is also chair of ocular pathology and program director of the Ophthalmic Pathology Fellowship in the Department of Pathology and Genomic Medicine at Houston Methodist Hospital. Financial disclosures: NASA: S.
Zélia Maria Corrêa, MD, PhD Ocular oncologist and The Tom Clancy Endowed Professor of Ophthalmology at the Johns Hopkins Wilmer Eye Institute in Baltimore. Financial disclosures: Castle Biosciences: C.
Deepak P. Edward, MD Professor of ophthalmology at the University of Illinois College of Medicine in Chicago. Financial disclosures: None.
Bita Esmaeli, MD Professor of ophthalmology in the Department of Plastic Surgery at the University of Texas MD Anderson Cancer Center in Houston. Financial disclosures: None.
Hans E. Grossniklaus, MD, MBA Interim Vice Chair of Emory Eye Center in Atlanta. He also holds the F. Phinizy Calhoun Jr. Professorship in Ophthalmic Pathology and is director of the L.F. Mont-gomery Pathology Laboratory, director of the Section of Ocular Oncology and Pathology, and senior professor of ophthalmology at Emory University. Financial disclosures: National Cancer Institute: S; NEI: S.
Tatyana Milman, MD Ophthalmic pathologist with the Ocular Pathology Service at Wills Eye Hospital in Philadelphia. She is also associate professor of ophthalmology and associate professor of pathology, anatomy, and cell biology at Thomas Jefferson University in Philadelphia. Financial disclosures: None.
David J. Wilson, MD Paul H. Casey Chair of Ophthalmology and the director of the Casey Eye Institute at the Oregon Health & Science University in Portland. Financial disclosures: None.
See the disclosure key at www.aao.org/eyenet/disclosures.