Download PDF
Could ophthalmologists eventually be able to protect glaucoma patients’ vision by giving them nutritional supplements that rev up the mitochondria in retinal ganglion cells (RGCs)? Results of a small phase 2 dosing and safety trial suggest that the answer might be yes.1
“This our first attempt at proof-of-concept in humans for this type of intervention in glaucoma,” said study leader Jeffrey M. Liebmann, MD, at Columbia University Medical Center in New York City. “So far, it looks like there’s a potential role for vitamin and nutritional supplementation as a treatment to help protect nerve cells.”
With this neurorecovery strategy, “you postulate that some RGCs are sick—and that if you provide them with neuroenhancing compounds, the cells will function a little bit better, and we’ll see a small uptick in visual function,” Dr. Liebmann added.
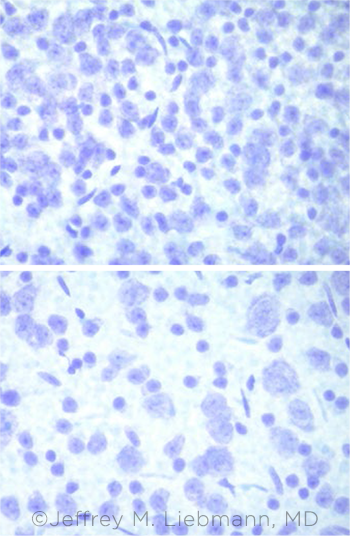 |
RGC LOSS. RGCs are plentiful in a healthy mouse (top). In comparison, the mouse with glaucoma (bottom) has experienced substantial RGC loss.
|
Two compounds tested together. In the study, 32 participants with treated open-angle glaucoma and moderate visual field loss were randomized to placebo or to oral doses of two compounds:
Nicotinomide is a vitamin B3 amide that is a precursor for nicotinamide adenine dinucleotide (NAD+). NAD+ is a key intracellular molecule involved in mitochondrial production of energy and in redox metabolism. As nicotinamide levels in sera decline with age, the investigators hypothesized that increasing the availability of nicotinamide (and, as a result, NAD+) might prevent flagging RGCs from progressing further toward apoptosis, Dr. Liebmann said.
Pyruvate is a compound that can overcome ocular deficits in glycolysis. It also acts as an antioxidant in the retina and aids oxidation and adenosine triphosphate production in RGCs and other cells. Animal studies have shown that levels of retinal pyruvate fall in response to high IOP and that this decrease occurs before detectable optic nerve degeneration.
Results. The participants were followed for a median of 2.2 months. Visual field tests performed during this time showed that the number of improving test locations was higher in the treatment group than in those who received placebo (p = .005).
Looking ahead. Buoyed by the early hints of efficacy, the investigators are planning to follow up with a larger, longer-term clinical trial to gauge the effectiveness of this strategy.
Dr. Liebmann noted that his patients, who often follow the glaucoma literature, have begun asking him about taking nicotinomide and pyruvate. “They want to know how much to take. But I try not to encourage too many people to use it, as our data are very limited. We will know much more in the next two years.” If pressed, Dr. Liebmann recommends nicotinamide 1,000 mg daily, divided into two equal doses. Although participants in the dosing trial received up to 3,000 mg daily with no serious adverse events, longer studies are needed to rule out downstream adverse effects with larger doses, he said.
—Linda Roach
___________________________
1 De Moraes CG et al. JAMA Ophthalmol. Published online Nov 18, 2021.
___________________________
Relevant financial disclosures: Dr. Liebmann—Allergan: C; Diopsys: O; Galimedix Therapeutics: C,O; Thea Pharmaceuticals: C.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Jeffery None.
Dr. Liebmann Allergan: C; Diopsys: O; Galimedix Therapeutics: C,O; Genentech: C; Infocus Capital Partners: C; NEI: S; Qura: C,O; Sustained Nano Systems: C,O,P; Thea Pharmaceuticals: C.
Dr. Rosenfeld Apellis: C,O; Bayer: C; Carl Zeiss: C,S; Boehringer Ingelheim: C; Chengdu Kanghong Biotech: C; Gyroscope Therapeutics: S; OcuDyne: C,O; Ocunexus (inflammX): C; Regeneron: C; Stealth BioTherapeutics: S; Unity Biotechnology: C; Valitor: O; Verana Health: O.
Dr. Wirta AbbVie: S; Aerie: C,S; Allergan: C,S; Allysta: S; Bausch + Lomb: S; Eyenovia: C,S; Jennivision: S; Nicox: S; Novaliq: S; Novartis: C,S; Ocuphire: S; Ora: S; Orasis: S; Osmotica: S; Oyster Point Pharma: C,S; Qlaris: S; Santen: C,S; TearCare: S; Tersus: S; Visus: S.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|
More from this month’s News in Review