By Linda Roach, Contributing Writer, interviewing Christine A. Curcio, PhD, K. Bailey Freund, MD, and Glenn J. Jaffe, MD
Download PDF
If you use high-resolution imaging to guide treatment of retinal diseases, the most important thing to know about outer retinal tubulation (ORT) structures is that they commonly appear in cross-sectional images as features that look somewhat like intraretinal cysts—but aren’t.
The tubular structures in ORT-affected eyes appear on high-resolution, spectral-domain ocular coherence tomography (SD-OCT) B-scans as well-defined circular or ovoid areas of hyporeflectivity, surrounded by a hyperreflective band, said K. Bailey Freund, MD, who coauthored the first clinical description of ORT.1
“If you did not know that these lesions existed, particularly if you were using the older time-domain OCT devices, you could easily confuse them with cysts or subretinal fluid,” said Dr. Freund, who practices in New York City. “And if you were using a treatment regimen that was guided by presence or absence of fluid, you could end up giving intravitreal injections to an eye that really didn’t need them.”
Many Roads Lead to ORT
Despite this narrow clinical utility, ORT has garnered increasing attention over the last several years because of the realization that tubulations in the outer nuclear layer represent a common neurodegenerative pathway in a variety of retinal diseases. These include neovascular age-related macular degeneration (AMD); geographic atrophy (GA) secondary to AMD and other disorders; inherited retinal diseases, particularly choroideremia; and mitochondrial diseases.2
Prognostic value. In all of these conditions, the presence of ORT structures indicates disorganized outer retinal layers, irreversible photoreceptor damage, and a worse visual prognosis, Dr. Freund said. “Most people would consider ORT a sign that, at least in that one particular area of the macula, you’re not going to be able to recover visual function,” he said.
A better understanding of the ORT process eventually might yield clues about how to stop photoreceptors from dying, said Glenn J. Jaffe, MD, at Duke Eye Center in Durham, North Carolina. In treatment trials, a biomarker like ORT might prove valuable for excluding prospective participants unlikely to regain vision, he said.
“The hope would be that we could help preserve people’s vision if we can prevent the degeneration of the photoreceptors,” Dr. Jaffe said. “Whether it’s with a neurotrophic agent or some other type of treatment, we are becoming more aware that we need to be able to prevent the loss of the photoreceptors in these diseases.”
Clues from CATT. Dr. Jaffe said he became interested in ORT because of what he observed during the Comparison Age-related Macular Degeneration Treatment Trials (CATT).3 “We were the OCT reading center for CATT, and while we were looking at the images I started to notice that ORT [structures] were becoming more frequent as the study went on. So we looked at the percentages and found that by 5 years, about 1 in 5 patients had this characteristic appearance,” Dr. Jaffe said.
 |
|
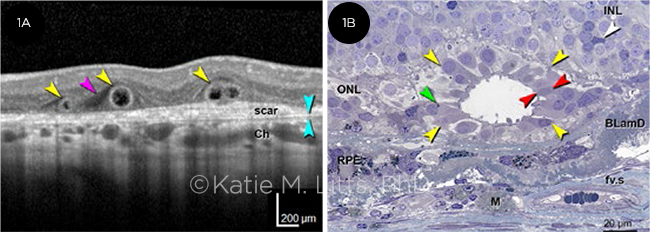
ORT ON OCT. (1A) Representative SD-OCT B-scan of 3 ORT cross-sections (yellow arrowheads) in an 81-year-old woman with neovascular AMD. Two closed ORT structures are evident on the left, and a branching tubulation can be seen on the right. A hyporeflective wedge (pink arrowhead) and Bruch membrane (cyan arrowhead) are also evident. (1B) High-resolution histology section of degenerate cones in ORT, at 1.5 mm from the fovea, in a different 81-year-old woman with neovascular AMD. Cone lipofuscin (green arrowheads), mitochondria in outer fiber (red arrowheads), Müller cell body (white arrowhead), and ORT cross-sections (yellow arrowheads) are indicated. BLamD = basal laminar deposit; Ch = choroid; fv.s = fibrovascular scar; INL and ONL = inner and outer nuclear layer; M = lipid-containing macrophage; RPE = entombed and melanotic retinal pigment epithelium. © Katie M. Litts, PhD. Invest Ophthalmol Vis Sci. 2016;57(6):2647-2656. Reprinted under Creative Commons License Attribution-NonCommercial-NoDerivatives 4.0 International License.
|
Genesis of Tubulation
The tubules characteristic of ORT are the retina’s heroic yet last-ditch attempt to protect localized areas of macular cone photoreceptors from the failure of the underlying retinal pigment epithelium (RPE), said Christine A. Curcio, PhD, at the University of Alabama at Birmingham.
“What ORT lesions have in common with each other is that the RPE is dying or is gone. These are responses of retina cells to the extreme stress of this detachment,” Dr. Curcio said.
In 1996, her research group published the first histological descriptions of photoreceptors surviving in mysterious interconnected tubes in the maculas of eyes with neovascular AMD.4 It wasn’t until 2009 that Dr. Freund and his colleagues, using SD-OCT images, published the first clinically oriented paper about “a peculiar outer retinal morphologic change occurring in a variety of advanced degenerative retinal disorders.”1
Since those publications, Drs. Curcio and Freund have worked together to correlate high-resolution histopathology in donor eyes with eye-tracked OCT images of living eyes, in order to understand the ORT process.2,5
Gliosis implicated. Their overall conclusion: Tubulation is a protective gliotic response—ultimately futile—triggered by activated Müller cells, Dr. Curcio said. “The Müller cells are trying to take care of the remaining photoreceptor cells. They are protecting them from the failure of the RPE and all the problems in the RPE/Bruch membrane complex.”
Dr. Freund agreed. “It’s the Müller cells that are driving this process, and in the very late stage of tubulation you only have Müller cells and no surviving cones,” he said.
Rolling up the photoreceptors. As described recently by Dolz-Marco et al.,2 ORT occurs when the external limiting membrane (ELM), which is normally a thin horizontal reflective line made by Müller cells and photoreceptors, begins “circling the wagons.” The ELM descends toward Bruch membrane and gradually begins scrolling the at-risk photoreceptors into a tubular structure. Depending on the stage of development, the outer band can appear in cross-section as flat, J-shaped, or partially (or fully) curved back on itself.
Both the inner and outer segments of the scrolled photoreceptors initially point radially into the lumen delineated by the ELM,2 and these can be seen clinically as a reflective fringe around the hyporeflective lumen (dark on the OCT).
As ORT progresses, the lumen becomes uniformly hyporeflective as the outer segments degenerate and the inner segments are pulled back across the ELM. Remnants of the numerous mitochondria in the inner segments migrate into the cell body of the degenerating cells, accounting for the reflectivity of the outer band of ORT.
In their study of 38 eyes with preexisting ORT, Dolz-Marco et al. found that the mean time for new tubules to form was 14.9 months.2 It is unknown how long the end-stage ORT lesions persist in the retina, but in clinical experience they commonly are stable through at least 3 years of follow-up.6
Serpentine patterns on OCT. En face OCT scans of ORT-affected maculas reveal the tubules snaking in various patterns across the retina. “A branching or pseudodendritic pattern is observed mainly in association with neovascularization, whereas a singular tube may line the border of GA. Interestingly, analysis of ORT over time has shown fluctuations in ORT volume in cross-sectional SD-OCT scans, even while the ORT footprint seen with en face imaging remains constant,” Dolz-Marco et al. wrote.2
What about GA? In a subgroup of affected patients in the Geographic Atrophy Treatment Evaluation (GATE) trial, ORT was present in 65% of eyes in the atrophic region and in 26% of eyes in the junctional zone.7 But there is disagreement over what this means, Dr. Jaffe said.
“It’s a little bit unclear” as to how well the presence of ORT predicts GA progression, he said. “In one of the publications that we’ve done, we found that it was associated with more rapid progression. But at least one other group [led by SriniVas Sadda, MD, at the University of Southern California] has reported not seeing that.”8
Attention to the different stages of ORT, which were not appreciated at the time of these prior studies, might help solve this discrepancy in future studies, Dr. Freund’s group suggested.2
___________________________
1 Zweifel SA et al. Arch Ophthalmol. 2009;127(12):1596-1602.
2 Dolz-Marco R et al. Ophthalmology. 2017;124(9):1353-1367.
3 Lee JY et al. Ophthalmology. 2014;121(12):2423-2431.
4 Curcio CA et al. Invest Ophthalmol Vis Sci. 1996;37(7):1236-1249.
5 Litts KM et al. Invest Ophthalmol Vis Sci. 2016;57(6):2647-2656.
6 Jung JJ, Freund KB. Arch Ophthalmol. 2012;130(12):1618-1619.
7 Moussa K et al. Retina. 2013;33(8):1590-1599.
8 Hariri A et al. Ophthalmology. 2015;122(2):407-413.
___________________________
Dr. Curcio is professor of ophthalmology and director of the AMD Histopathology Lab at the University of Alabama at Birmingham. Relevant financial disclosures: Heidelberg Engineering: S.
Dr. Freund practices at Vitreous Retina Macula Consultants of New York and is clinical professor of ophthalmology at the New York University School of Medicine in New York City. Relevant financial disclosures: Heidelberg Engineering: C; Optovue: C; Spark Therapeutics: C.
Dr. Jaffe is the Robert Machemer Professor of Ophthalmology and chief of the Vitreoretinal Division at Duke University and director of the Duke Reading Center at the Duke Eye Center in Durham, N.C. Relevant financial disclosures: Heidelberg Engineering: C.
For full disclosures and the disclosure key, see below.