Download PDF
Cracking the conundrum of nonadherence: A look at potential solutions, including novel drug delivery options.
The modern glaucoma drug formulary provides highly effective therapy for patients with glaucoma. Yet these vision-saving eyedrops have a significant drawback: The medication must get into the eye to work, an accomplishment that is easier said than done.
“You can develop all the best medicines in the world,” said Alan L. Robin, MD, at the University of Michigan in Ann Arbor and John Hopkins University in Baltimore. “But if you don’t take them, or you don’t use them properly so that they actually touch your eye, they are worthless.”
The challenge of patient adherence is not new. A study published more than 30 years ago discussed an electronic eyedrop monitor that could prove useful in assessing compliance with topical ocular therapy.1 And close to a decade ago, David S. Friedman, MD, MPH, PhD, at Wilmer Eye Institute in Baltimore, and his colleagues found that nearly 45% of patients using an electronic monitoring device who knew they were being monitored (so they would be on their best behavior) and who received free travoprost (so that they wouldn’t skimp on drops) used their eyedrops less than 75% of the time.2
In Dr. Friedman’s study, not only did patients report far higher medication use than their actual behavior, but the physicians also had difficulty identifying which patients were poorly adherent based on self-reporting or other subjective clues.
“The medical literature continues to raise awareness about the problem of adherence,” said Steven J. Gedde, MD, at the Bascom Palmer Eye Institute in Miami. “Many of our patients are not using their eyedrops correctly, and we need to take measures to improve adherence and use alternative treatments when necessary.”
A Multifactorial Challenge
The reasons for nonadherence to glaucoma drops are complex and multifactorial, and they vary from patient to patient. According to Tony Realini, MD, MPH, at the West Virginia University Eye Institute in Morgantown, nonadherent patients can be divided into 2 groups based on their underlying motivation.
Unintentional nonadherence. Some patients with glaucoma simply cannot place the drops in their eyes. There may be physical issues, including tremors, arthritis, and limited mobility, or cognitive issues, such as Alzheimer’s or simple forgetfulness. In addition, “Approximately 17% of glaucoma patients are reliant on others for their drops,” Dr. Robin said. “And these caretakers also may experience difficulty getting the drops into patients’ eyes.”
Other patients simply have no clue how to use drops correctly, even though they think they are being successful. Dr. Robin and his colleagues were among the first to videotape patients self-instilling eyedrops.3
The researchers found that only one-third of the study patients could successfully instill a single drop without touching the bottle tip to the eye or the ocular adnexa. Yet 92.8% of these patients reported no problem putting in their eyedrops, and 61.9% believed that they never missed their eye when administering the drop.
Instilling more than 1 drop in the eye per dose can also lead to nonadherence: Glaucoma medicine is filled based on estimates of how long a bottle of eyedrops should last. “Patients will run out before they can refill their prescription, and the pharmacy benefit manager will not approve another bottle until the next month,” Dr. Robin said. “These patients often cannot afford the out-of-pocket costs, so they go without their medicine.” (See “Tackling the Refill Dilemma.”)
Janet B. Serle, MD, at Mount Sinai School of Medicine in New York City, added, “The fact that the costs of these drugs can be prohibitive—a drugstore recently charged a patient $1,600 for a 90-day supply of 2 medications—has made running out of the drugs more problematic.
“Years ago, if patients could not get a full month out of their bottle, we could help them,” Dr. Serle added. “But sampling in the academic medical centers is no longer allowed, reducing our options. When patients run out of drops, it is frustrating both for us and for our patients.”
Intentional nonadherence. As Dr. Realini noted, “Glaucoma has no symptoms, so for some patients there is no obvious incentive to continue taking their drops, even when they are told they could go blind. Study results have shown that the severity of the disease and potential consequences of nonadherence do not matter to patients. For example, adherence to antirejection drugs after kidney transplant is also abysmal.”
Dr. Gedde agreed, likening the asymptomatic aspect of glaucoma to that of high cholesterol and high blood pressure. “If patients are not feeling well from an ocular standpoint—for example, with an eye infection—they are willing to take an antibiotic that will likely make them feel better. In contrast, glaucoma is generally an asymptomatic disease, and patients are less compelled to use medications when they are feeling well.”
Certain patients are simply fatalistic, Dr. Robin said. Some don’t believe that they will lose their vision and thus think drops are unnecessary. Others say, “I will go blind anyway, so why bother.”
 |
|
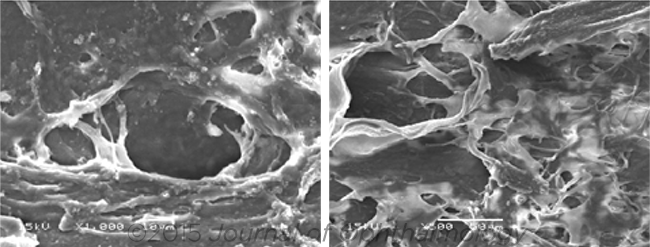
LASER. These images show the impact of ALT (left) and SLT (right) on the human trabecular meshwork tissue. Either procedure may be appropriate for selected nonadherent glaucoma patients.
|
Recognizing the Problem
To address nonadherence, ophthalmologists first need to recognize it, which is not always an easy task.
Red flags in the office. Dr. Gedde put forth a not-uncommon scenario: A patient in the clinic presents with excellent pressure, but his or her glaucoma continues to worsen.
“When ophthalmologists see progressive disease despite what appears to be good pressure control, nonadherence should be included in the differential diagnosis,” Dr. Gedde said. He compared the situation to flossing for a week before seeing the dentist for a 6-month checkup and then sheepishly admitting to avoiding flossing for the past 5 months. “Most patients will be honest about their medication use, if you ask in a nonjudgmental way,” Dr. Gedde explained.
Another possible sign of nonadherence is the inability to control a patient’s intraocular pressure (IOP) after trying different medications. “After the patient uses 1 agent after another with no response, you start wondering if the patient is actually taking the medication or instilling it properly. Watching the patient administer his or her drop can provide valuable insight,” Dr. Gedde said.
Red flags via EHRs. Electronic health records (EHRs) can be used to help track pharmacy refills and indicate when a patient is not regularly ordering his or her drops. “But EHRs will only help us if they are global and access information across the patient’s entire medical activity,” Dr. Realini warned. “For example, some university medical centers are closed systems: The EHR will only reflect the drugs refilled at the university pharmacy, not refills ordered in a community setting.”
 |
|
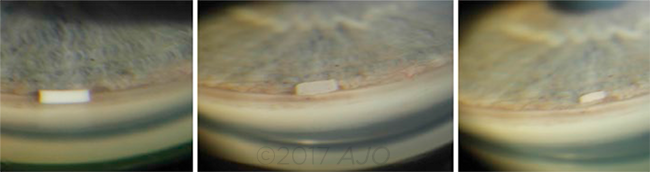
IMPLANT. These images are of the bimatoprost SR implant in a patient with open-angle glaucoma. (Left to right) The implant at 2 weeks, 9 months, and 12 months after injection.
|
Strategies to Improve Adherence
Dr. Friedman noted that ophthalmologists are under pressure to examine their patients efficiently and effectively, and tight schedules make it difficult to explore whether an individual is taking his or her drops.
Effective communication. “As doctors, we ask questions such as ‘Are you taking your drops correctly?’ This sounds judgmental and may not elicit a truthful response,” Dr. Friedman said. “One of the big changes in my practice is to create a nonjudgmental environment in which the questions are nonthreatening and compassionate.”
For example, Dr. Friedman will state, “It’s hard to take drops every day,” and wait to hear what the patients says. Or he will say, “Tell me about your eyedrops,” which often prompts patients to open up about their challenges. And after a few questions, he will ask, “How often do you think you are missing your drops?”
Dr. Friedman explained that this line of questioning makes it acceptable for patients to admit their difficulties. “Doctors should be conducting these conversations,” he said.
Dr. Robin agreed, suggesting that a crucial step in increasing adherence rates is to improve physician-patient communication. “I don’t want to blame doctors, but I don’t think doctors talk to patients as well as they should. We aren’t trained in medical school or residencies to explain drop instillation, for example, and we need to develop better tools to help doctors instruct patients.”
And there is evidence that communication can make a difference. Dr. Robin and his colleagues published a study showing that when physicians educated patients about how to administer their drops, there was a significant association with increased adherence and whether the patient took the correct number of doses each day.4
Smart technology. For patients who are forgetful, the ever-present smartphone can be helpful. “A smartphone won’t put a drug in your eye, but it can help you remember,” Dr. Robin said.
A study by Dr. Friedman and his colleagues confirmed this observation.5 In the study, which utilized telecommunication-based reminders linked to personal health records, patients using once-daily drops set up an automated telecommunication-based reminder system (call or text). Seventy nonadherent patients were randomized to either the automated-response (n = 38) or to the control group (n = 32).
The researchers found that the median adherence rate in the intervention group increased from 53% to 64% (and even higher, 73%, when comparing the participants who successfully completed the study after randomization), while there was no statistical change in the control group.
“Technology exists to help patients remember to take their drops,” Dr. Friedman said. “We need to help them tap into this resource.”
Tackling the Refill Dilemma
The Academy and the American Glaucoma Society (AGS) are very concerned about inadequate access to necessary medication for chronic glaucoma treatment. In 2009, and again in 2010, the 2 organizations approached the oversight committee for the Medicare Part D drug plans and were able to effect changes that resulted in an easing of the refill restrictions.
Medicare Part D drug plans now allow an override of the refill limits when patients seek to refill their eyedrop prescription at 70% of the predicted days of use (e.g., at day 21 for a 30-day supply). In addition, physicians can now request authorization for even earlier refills for patients in need.
A number of states have passed similar drop refill laws.
|
Rethinking Drug Delivery
One solution to the adherence challenge is to take the process of drug administration out of the patient’s hands entirely. Several novel drug delivery systems intended to do precisely that are under investigation.
In phase 3 trials. The following options are being investigated.
Bimatoprost SR Implant (Allergan). This biodegradable device is placed into the anterior chamber of the eye using a prefilled, single-use applicator system that releases bimatoprost over an extended period. Dr. Serle noted the device is currently undergoing phase 3 clinical trials. A phase 2 dose ranging study showed that the bimatoprost SR implant sufficiently lowered IOP in 95% of glaucoma patients at 12 weeks and 92% of glaucoma patients at 16 weeks. IOP reductions were similar to topical bimatoprost 0.03% through 12 weeks after administration, with no serious adverse events reported.6
“In the ongoing phase 3 trials, the implant is replaced every 16 weeks for a total of 3 times and the patients are being followed through 20 months after the initial administration,” Dr. Serle said. “It appears the eye tolerates the implant well.”
Bimatoprost Ocular Ring (Allergan). This technology involves a thin silicone ring suffused with bimatoprost that is slowly released over a 6-month period.
In a phase 2 clinical trial published by Brandt et al.,7 64 patients received the bimatoprost ring and artificial tears twice daily, and 66 patients in the control group wore an insert treated with no drug but used 0.5% timolol drops twice a day.
Eye pressure in the bimatoprost group fell 3.2 to 6.4 mm Hg over 6 months, in comparison to 4.2 to 6.4 mm Hg for the timolol group. Overall, eye pressure decreased in the group wearing the bimatoprost ring by ≥ 20% from the initial measurements over 6 months. The device was well-tolerated and safe, with a high retention rate of 90% for both groups at 6 months. A 13-month open-label extension study demonstrated similar IOP lowering to the last 3 months of the 6-month study, with a retention rate of 95%.8
“This appears to be an excellent option for patients seeking a less-invasive device, and a phase 3 clinical trial is ongoing,” said Dr. Serle.
Travoprost Punctal Plugs (Ocular Therapeutix). These plugs use the company’s polyethylene glycol hydrogel technology to release travoprost over a sustained period. At the end of the treatment period, the bioabsorbable plug begins to degrade and exits the nasolacrimal system.
Dr. Serle noted that phase 3 clinical trials are currently under way. However, she said, “There is very little information to share at this point.”
In earlier research stages. Other options under investigation include the following.
Intraocular devices. The iDose (Glaukos), a titanium implant similar to the iStent (a microinvasive glaucoma surgery device), is designed to continuously elute travoprost. The iDose is preloaded into an injector and inserted intracamerally into the anterior chamber angle. Phase 2 trials are ongoing.
The ENV515 Travoprost XR (Envisia Therapeutics), a biodegradable implant, delivers travoprost for more than 6 months with a single intraocular dose.
The NT-501 (Neurotech), an intraocular implant that is in phase 2 clinical testing, releases ciliary neurotrophic factor. The goal of this implant is neuroprotection (in contrast to other devices, which are designed to lower IOP).
Extraocular devices. Latanoprost-Eluting Contact Lenses (Massachusetts Eye and Ear) contain a thin film of latanoprost-encapsulated polymers in the periphery to deliver medication gradually. The lenses are designed to be worn for 7 days. In a preclinical study, the lenses proved to be at least as effective as daily latanoprost eyedrops.9
The TODDD (Topical Ophthalmic Drug Delivery Device), developed by VISTA Scientific, is a soft flexible device that floats on the tear film and can simultaneously deliver several different ocular hypotensive medications.
The Bionode Platform (Purdue Research Foundation) involves a standard contact lens that has been fitted with a gold trace. The gold trace receives an electromagnetic field from a specially equipped pair of glasses. The electromagnetic field is converted into a current that stimulates the muscles around the Schlemm canal, somewhat analogous to pilocarpine, and the proposed mechanism is enhanced trabecular outflow.
Sustained-release versus eyedrops. Dr. Realini noted that the primary issue facing the viability of these novel options is not necessarily the technology. Instead, it’s whether drug companies can demonstrate the value of the devices when they are compared to currently available medications.
“For these devices to be covered by payers, the pharmaceutical companies may need to provide better outcome data to clarify the benefits of sustained delivery in nonadherent patients,” Dr. Realini said. “As a prescriber, I want compelling evidence that they improve outcomes, given their additional costs to both the patient and the health care system.”
 |
|
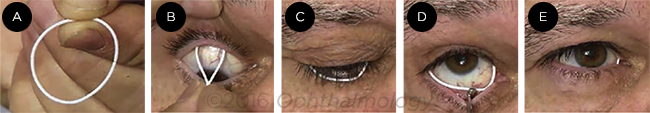
RING. The bimatoprost ring (A) releases a descending dose of medication over a 6-month period. (B) The top half is placed in the upper fornix, and (C) the lower lid is gently retracted so that (D) the lower half can be seated in the lower fornix. After placement (E), a small portion of the insert is visible in the medial canthus.
|
Opting for Laser or Surgery
Dr. Gedde noted that while he is optimistic about the future of sustained-release and other innovative drug delivery systems, he has found that laser trabeculoplasty is a good option for his nonadherent patients with open-angle glaucoma.
A number of studies support the efficacy and safety of 2 different types of lasers, Dr. Gedde noted—argon laser trabeculoplasty (ALT) and selective laser trabeculoplasty (SLT).
“A majority of patients will have a beneficial response to laser trabeculoplasty, and I believe this is a safe procedure and a particularly useful treatment option for the patient who is nonadherent with glaucoma medical therapy,” Dr. Gedde said.
Dr. Realini agreed, adding that in most patients, he recommends SLT for their first intervention, which is a covered procedure in his practice. “When you factor in nonadherence, primary SLT makes sense as a cost-effective alternative.”
Surgical options are also available for patients whose IOP cannot be controlled with medical and laser therapy, Dr. Gedde said.
 |
|
CONTACT LENS. Bionode’s contact lens uses electrical impulses to normalize IOP.
|
A Forever Disease
The challenge of adherence is not confined to the field of ophthalmology. “Changing behaviors is one of the greatest challenges in modern medicine,” noted Dr. Friedman. To counter problematic behavioral patterns in ophthalmology, he said, the topic of preventing vision loss must be the primary focus of the patient visit. “Glaucoma is a forever disease,” Dr. Robin said. “We need to help patients understand that and focus on promoting adherence throughout their lives.”
___________________________
1 Kass KA et al. Arch Ophthalmol. 1984;102:1550-1554.
2 Okeke CO et al. Ophthalmology. 2009;116(2):191-199.
3 Stone JL et al. Arch Ophthalmol. 2009;127(6):732-736.
4 Sleath B et al. Ophthalmology. 2015;122(4):748-754.
5 Boland MV et al. JAMA Ophthalmol. 2014;132(7):845-850.
6 Lewis RA et al. Am J Ophthalmol. 2017;175:137-147.
7 Brandt JD et al. Ophthalmology. 2016;123(8):1685-1694.
8 Brandt JD et al. Ophthalmology. 2017. doi:10.1016/j.ophtha.2017.04.022. Published online May 17, 2017.
9 Ciolino JB et al. Ophthalmology. 2016;123(10):2085-2092.
Meet the Experts
David S. Friedman, MD, MPH, PhD Director of the Dana Center for Preventive Ophthalmology and the Alfred Sommer Professor of Ophthalmology at the Wilmer Eye Institute in Baltimore, and professor of epidemiology and international health at the Johns Hopkins Bloomberg School of Public Health in Baltimore. Relevant financial disclosures: Alcon: C; Allergan: C; ForSightV: C.
Steven J. Gedde, MD Professor of ophthalmology, vice chairman of education, and director of the residency training program at the Bascom Palmer Eye Institute in Miami. Relevant financial disclosures: None.
Tony Realini, MD, MPH Professor of ophthalmology and director of the Glaucoma Service, clinical research, and the Glaucoma Fellowship at West Virginia University Eye Institute in Morgantown, W. Va. Relevant financial disclosures: None.
Alan L. Robin, MD Professor of ophthalmology at the University of Michigan in Ann Arbor, Mich., associate professor of ophthalmology at the Wilmer Eye Institute in Baltimore, and associate professor of international health at Johns Hopkins Bloomberg School of Public Health in Baltimore. Relevant financial disclosures: Aerie: C; DSM: C; Novaliq: C; Novartis: C.
Janet B. Serle, MD Professor of ophthalmology; director of the Glaucoma Clinical and Research Fellowships; and director of the Glaucoma Clinical Research Laboratory and the Preclinical Glaucoma Research Laboratory at Mount Sinai School of Medicine in New York. Relevant financial disclosures: Aerie: C; Allergan: C,S; Bausch + Lomb: C; Inotek Pharmaceuticals: S; Ocular Therapeutix: S.
|