By Mary Labowsky, BS, Morgan Godin, MD, and M. Tariq Bhatti, MD
Edited by Steven J. Gedde, MD
Download PDF
While decorating her Christmas tree with her 2 young children, 26-year-old Veronica Cannon* noticed that the ornaments seemed less vibrant than she remembered. In fact, she had trouble telling the red and green ones apart. Looking through her eyes one at a time, she realized that while the vision in her left eye was normal, through her right eye, the ornaments appeared gray.
For the next few days, the vision in her right eye continued to be “grayed out” and blurry in certain areas. She also developed eye pain with movement of her right eye. She went to her general practitioner who, suspecting optic neuritis, referred her to a neurologist. The neurologist recommended an MRI of the brain and orbits with contrast, thyroid studies, and markers of inflammation (erythrocyte sedimentation rate [ESR] and C-reactive protein [CRP]) and autoimmunity (antinuclear antibody [ANA] and rheumatoid factor). Her blood work was found to be unremarkable, and the MRI was normal with no optic nerve enhancement. The ocular pain spontaneously resolved over the next week; her visual disturbances in the right eye persisted but did not progress.
Then several weeks later, she woke up one morning to find that she was unable to perceive color well with her left eye. She had ocular pain similar to that which she had experienced previously in her right eye. At this time, Ms. Cannon was referred to our clinic for further evaluation of suspected recurrent optic neuritis.
 |
|
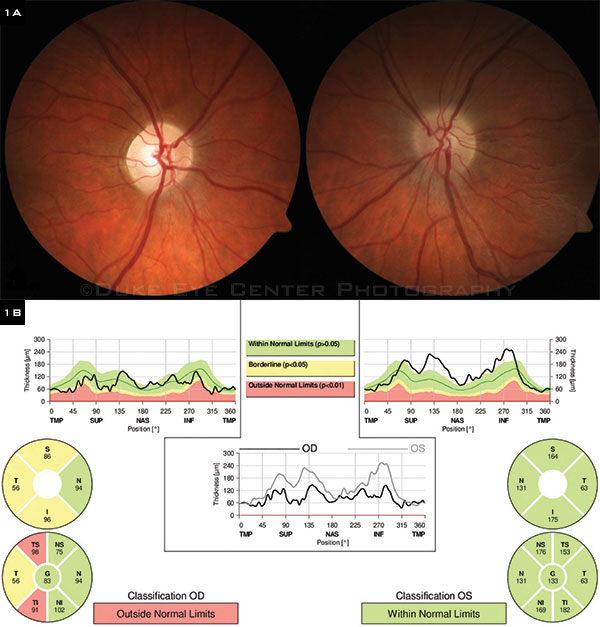
What’s Your Diagnosis? (1A) At presentation, we noted temporal pallor of the right optic disc and nasal edema of the left. (1B) OCT showed temporal thinning of the right peripapillary retinal nerve fiber layer (RNFL) and mild nasal thickening of the left.
|
We Get a Look
When we met Ms. Cannon, she described symptoms of color desaturation and blurred vision in the inferior hemifield of her left eye, as well as residual patchy color deficits and blurred vision in her right eye. She reported intermittent “brain-freezes” and chronic fatigue but otherwise had no associated symptoms. She denied having focal neurologic deficits, including Lhermitte’s sign and Uhthoff’s phenomenon. Furthermore, she denied any recent illnesses, animal exposure, tick bites, joint pain, or skin rashes. She had an unremarkable ocular history. Her past medical history was notable only for stable hypothyroidism secondary to Hashimoto thyroiditis.
 |
|
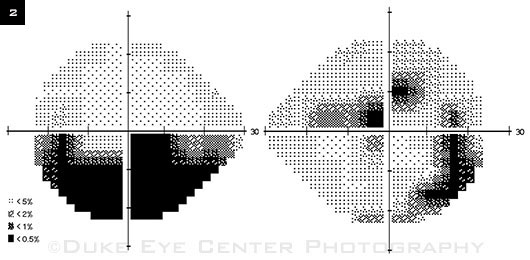
Visual Fields. We noted defects in both eyes.
|
What We Found
On examination, her vision was 20/15 in the right eye and 20/20 in the left eye. She correctly identified 10 of 10 Ishihara color plates bilaterally. Pupillary light reactions were brisk and equal, with no relative afferent pupillary defect. Motility exam demonstrated full ocular movements, though she reported eye pain bilaterally with extremes of gaze. Her visual fields were intact to confrontation on the right but depressed inferotemporally on the left. Her slit-lamp examination and intraocular pressures were normal. Dilated fundus exam revealed a sharply demarcated optic disc with temporal pallor on the right and nasal optic disc edema with trace elevation on the left (Fig. 1A). Optical coherence tomography (OCT) showed that the peripapillary retinal nerve fiber layer was thinned temporally in her right eye and thickened nasally in her left eye (Fig. 1B). Automated visual field testing demonstrated double arcuate defects in the right eye and an inferior arcuate defect in the left eye with extension to the periphery (Fig. 2). Fluorescein angiography demonstrated late leakage of the left optic disc.
(click to expand)
Narrowing Our Differential Diagnosis
Ms. Cannon’s presentation with 2 sequential episodes of blurred vision, color desaturation, and painful ocular movements (first in one eye, then the other) was concerning for optic neuritis. Her funduscopic exam and OCT results seemed consistent with our suspicion. We ordered more blood tests to rule out infectious etiologies of optic neuritis, including Bartonella, Lyme disease, syphilis, and tuberculosis, all of which returned negative.
Demyelinating disease. In a woman of childbearing age, recurrent episodes of optic neuritis should raise concern for a central demyelinating disorder such as multiple sclerosis (MS) or neuromyelitis optica (NMO). We thus repeated the MRI of the brain and orbits and added an MRI of the cervical spine and a test for NMO antibody. These tests were all negative. We offered a lumbar puncture to be thorough, but the patient declined.
We were faced with a diagnostic dilemma: What was causing our patient’s visual loss? We took a step back and asked an even more basic question: At which anatomic level was the visual loss taking place?
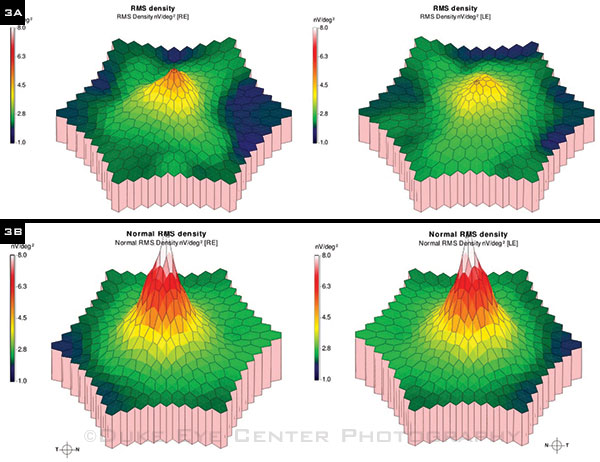
Retina vs. optic nerve. Involvement of her optic nerves was apparent from the fundus exam and OCT. To investigate the possibility of concurrent retinopathy, we ordered a multifocal electroretinogram (mfERG), which turned out to be abnormal (Fig. 3A). It became clear to us that her retina and optic nerves were both affected by the disease process.
Paraneoplastic vs. non-paraneoplastic autoimmunity. Given her history of autoimmune disease (Hashimoto thyroiditis) and her demographic risk factors (female sex and young age), we suspected an autoimmune process. We analyzed her blood for the presence of antiretinal and anti–optic nerve antibodies. The test returned positive for antibodies against retinal GAPDH, aldolase, arrestin, and optic nerve 35-40 kDa proteins. The presence of these antibodies confirmed an autoimmune process, though none were specific for a particular syndrome (Table 1). Next, it was imperative to determine whether this autoimmunity was primary or secondary to an underlying neoplasm. We undertook an extensive malignancy workup, looking specifically for lung, gynecologic, and hematologic cancers. Computed tomography of the chest, abdomen, and pelvis returned normal. A complete blood count, mammogram, and recent Pap smear were also normal.
Diagnosis. We concluded that our patient had autoimmune-related retinopathy and optic neuropathy (ARRON; see Table 1). The negative results of her malignancy workup were critical for making this diagnosis.
 |
|
mfERG. (3A) We noted bilateral decreased peaks in retinal response density indicative of retinal dysfunction. (3B) A normal topographic mfERG for reference.
|
Discussion
Though quite rare, a retinopathy or an optic neuropathy can be a manifestation of an underlying carcinoma (i.e., paraneoplastic). Ocular paraneoplastic syndromes may arise in patients with known cancer, but they can also serve as a harbinger of occult malignancy.1
Cancer-associated retinopathy (CAR) is the most common ocular paraneoplastic syndrome. It presents as progressive, painless vision loss with varying degrees of cone and rod dysfunction.1 Cone dysfunction manifests as impaired central vision, dyschromatopsia, and hemeralopia accompanied by photosensitivity and glare. Rod dysfunction presents with decreased peripheral vision, poor dark adaptation, and nyctalopia. In approximately 50% of cases, CAR precedes the diagnosis of cancer. CAR can occur with many different cancers but most commonly is associated with lung (small cell), breast, and gynecologic cancers.1 The associated autoantibodies are believed to have specific pathologic and prognostic significance (Table 1).
Melanoma-associated retinopathy (MAR) can manifest years after the diagnosis of a cutaneous melanoma and may reflect cancer progression.1 Autoimmunity against retinal bipolar cells mediates an acute onset of vision loss with features of rod dysfunction.1 MAR is also associated with several autoantibodies, but there is little consensus on their pathologic relevance.
Paraneoplastic optic neuropathy (PON) primarily affects women and is frequently associated with small cell lung cancer.1 It often presents as a bilateral, subacute, painless, and progressive visual loss described as blurriness, dimness, or loss of peripheral vision.1 The presentation of PON is variable, but a suggestive clinical triad includes optic neuritis, vitreous cells, and retinal vessel leakage.1 It is associated with antibodies against the collapsin-response mediated protein (CRMP-5), which is involved in embryologic development across the nervous system. Therefore, PON is often seen in conjunction with other neurologic manifestations.1
ARRON. Only when a battery of tests fails to uncover a malignancy can one diagnose the non-paraneoplastic syndrome ARRON. The presentation of ARRON ranges from normal visual acuity (VA) to no light perception.1 Exam findings include optic disc pallor as well as nonspecific retinal changes and abnormalities on ERG. Though it has many associated autoantibodies, it is deemed “autoimmune-related” because it remains unknown whether these antibodies are an epiphenomenon or truly pathogenic.2 Antiretinal antibodies similar to those associated with ARRON can be found in other conditions such as retinitis pigmentosa, cystoid macular edema, and Behçet syndrome.2 Furthermore, expression of antiretinal antibodies can occur in normal individuals.3
Treatment options for ARRON include immunosuppression, plasma exchange, and intravenous immunoglobulin.4 Many experts speculate that ARRON may not be a single disease but rather a placeholder for an eventual paraneoplastic diagnosis such as CAR, MAR, or PON. The time from diagnosis of presumed autoimmune retinopathy to a cancer diagnosis has been reported to occur as long as 11 years after the visual loss.3 In contrast, 1 report describes a patient with autoimmune-related retinopathy who lived more than 3 decades without developing cancer.1 Until diagnostic modalities can decisively differentiate these syndromes, the management of ARRON should include not only immunosuppression but also diligent, lifelong surveillance for malignancy.
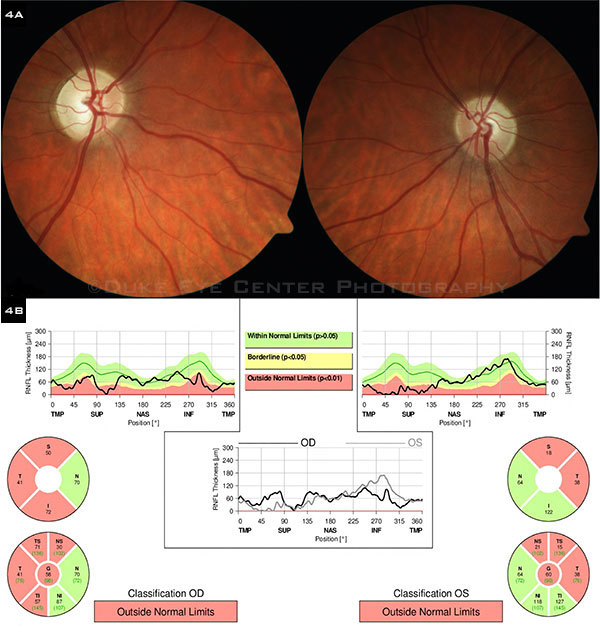 |
(4A) Bilateral temporal pallor of the optic nerves can be seen in these fundus photos, taken 6 months after the initial presentation in our clinic. Note that the left disc edema (see Fig. 1) has been replaced by pallor. (4B) OCT of the RNFL demonstrates bilateral temporal and superior thinning of the optic nerves.
|
Our Patient’s Progress
Following the diagnosis, we initiated treatment with a 3-day course of intravenous corticosteroids that unfortunately did not reverse her visual field deficits, though our patient’s VA has remained 20/20 in both eyes. Funduscopic exam at subsequent visits demonstrated resolution of her left optic disc edema with development of temporal pallor (Fig. 4). She has had no further episodes of visual loss, and has remained cancer free. Given the stability of her disease and preserved VA, she is not currently undergoing any medical treatment, though we monitor her every 3 months.
___________________________
* Patient name is fictitious.
___________________________
1 Rahimy E, Sarraf D. Surv Ophthalmol. 2013;58(5):430-458.
2 Braithwaite T et al. Autoimmun Rev. 2014;13(4-5):534-538.
3 Saito W et al. Arch Ophthalmol. 2007;125(10):1431-1433.
4 Oyama Y et al. J Neuroophthalmol. 2009;29(1):43-49.
___________________________
Ms. Labowsky is a medical student; Dr. Godin is a resident; and Dr. Bhatti is professor of ophthalmology, professor of neurology, and chief of neuro-ophthalmology; all 3 are at Duke University in Durham, N.C. Relevant financial disclosures: None.
For full disclosures and the disclosure key, see below.