By Jean Shaw, Senior Editor, interviewing Rebekah A. Braslow, MD, Sang Jin Kim, MD, and Michael F. Marmor, MD
Download PDF
Despite the advent of newer drugs, hydroxychloroquine (HCQ) continues to be a mainstay in the treatment of systemic lupus erythematosis (SLE), rheumatoid arthritis (RA), and other connective tissue diseases. Moreover, it is used as an adjunct in chemotherapy, and it is being investigated as a treatment for diabetes and heart disease, thanks to its anti-inflammatory, lipid-lowering, and antithrombotic properties.1
But as ophthalmologists know, excessive HCQ dosages can result in toxic damage to the eye. In an effort to reduce the incidence of HCQ retinopathy, the Academy published screening guidelines in 2002. These were updated in 2011 and again last year.2
Here’s an overview of the latest guidelines—and troubling evidence that far too many patients are still receiving too high a dose of HCQ (see “Excessive Dosing Still a Problem,” below).
Rethinking Risk
Highlights of the 2016 guidelines include the following.
Use real body weight. “The bottom line is that the daily dose should be 5.0 mg per kg or less, using real body weight,” said Michael F. Marmor, MD, at Stanford University in Palo Alto, Calif.
This represents a significant change from the 2011 guidelines, which recommended using ideal body weight to determine dosages. The change was prompted in part by a 2014 study of 2,361 people who had used HCQ continuously for at least 5 years. In this study, Dr. Marmor and his coauthor, Ronald B. Melles, MD, found that real body weight was a better predictor of the risk of toxicity.3
One problem with using ideal body weight to determine HCQ dosages was that doing so placed smaller patients at risk of being overdosed, said Dr. Marmor. “These connective tissue diseases disproportionately affect women, and many of them are very slight in stature.”
Using real body weight “corrects the problem of overdosing the smaller women and is equally good as a predictor across a broad range of body types,” he said. As a practical bonus, the guideline of 5 mg/kg is much easier to calculate, he added.
Adjusting doses. HCQ only comes in 200-mg tablets, so how does one prescribe the proper dose? Dr. Marmor points out that blood levels of HCQ stabilize slowly, so the weeklong dose can be achieved by varying the number of pills on different days of the week.
Think dose plus duration. Dose is only part of the equation, however. “Risk is a function of daily dose plus length of time,” said Dr. Marmor.
Patients who have been taking HCQ for 5 or more years are at increased risk of developing HCQ retinopathy, even if they have no other risk factors. For instance, in the 2014 study, the risk of HCQ retinopathy remained low during the first 10 years of use (less than 2%), even for patients who took the recommended dose of 4.0-5.0 mg/kg of the drug—and then rose to almost 20% after 20 years of use.3
What about cumulative dose? “We used to think in terms of cumulative exposure to HCQ,” Dr. Marmor said. Specifically, 1,000 g of cumulative exposure was considered the cutoff. “But that just doesn’t hold up any more,” he said, “because some patients will take smaller or larger daily amounts. Risk depends on the balance of dose/kg with duration of use.”2,3
Consider additional risk factors. Other risk factors include the following.
Renal disease. “The big risk factor that complicates things is kidney disease,” Dr. Marmor said. Because HCQ is cleared by the kidneys, renal disease raises the risk of toxicity, and both dosage and screening frequency may need to be adjusted in these patients.2
Tamoxifen use. Concomitant use of HCQ and tamoxifen, which is prescribed to treat and prevent breast cancer, raises the risk of HCQ toxicity approximately 5-fold. “Tamoxifen is also retinotoxic, and there may be some metabolic synergy,” Dr. Marmor said. Thus, patients who are taking HCQ and tamoxifen concomitantly need to be carefully screened.
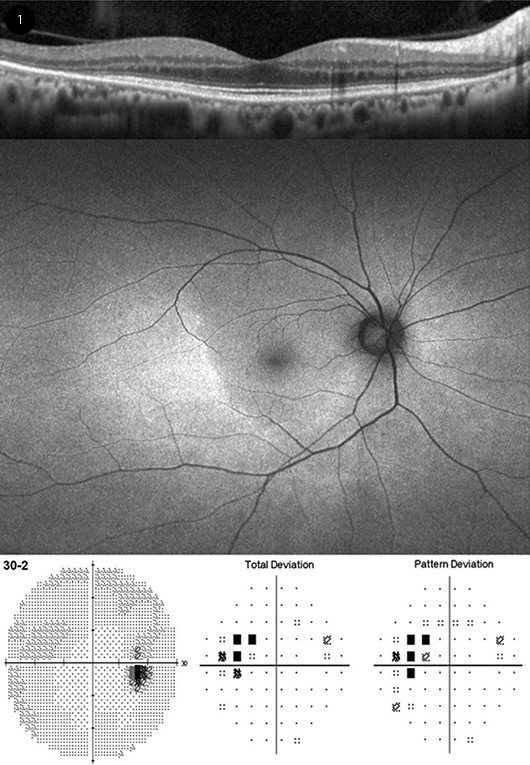 |
|
PERICENTRAL PRESENTATION. (Top) Horizontal spectral-domain optical coherence tomography, showing temporal loss of the outer retina (ellipsoid zone and interdigitation zone). (Middle) Wide-field fundus autofluorescence showing a broad area of hyperfluorescence extending beyond the outer edge of the inferotemporal macula. (Bottom) 30-2 visual field (VF) with superonasal scotoma corresponding to the retinal changes. A 10-2 VF test showed normal results.
|
Nuances in Presentation
One startling fact that has recently emerged is that HCQ retinopathy tends to present atypically in Asian patients.
“While most patients of European descent show initial photoreceptor damage in the classic parafoveal distribution, most patients of Asian descent will show initial damage in a more peripheral extramacular distribution (Fig. 1) near the arcades,” the 2016 guidelines explain.2
In his own practice, Dr. Marmor said, “We’re in Northern California, and we began to realize that there’s a different pattern of damage in Asian patients—that we were at risk of missing early toxicity further out.”
In a retrospective study published at the end of 2014, Drs. Marmor and Melles found that 50% of California patients of Asian heritage who had HCQ retinopathy showed degenerative changes near the vascular arcades rather than in the “typical” parafoveal region (and another 30% showed a mix of parafoveal and pericentral damage).4 Two parallel studies on Korean patients, one of which was published earlier this year, showed similar prevalence of a pericentral pattern.5,6
“Pericentral retinal damage seems more common in Asian patients,” commented Sang Jin Kim, MD, a coauthor of the 2017 study. In that series, 9 of 174 patients who had taken HCQ for more than 5 years (5.2%) had HCQ retinopathy.6 And of those 9 patients, Dr. Kim said, 6 [66.7%] “were determined to have a pericentral or mixed pericentral and parafoveal pattern.”
The question of why this is the case remains unanswered at present. “We haven’t the foggiest idea,” Dr. Marmor said. “We presume that it’s genetic.”
Screening Recommendations
Because HCQ retinopathy cannot be reversed, proper screening is critical. The 2016 guidelines recommend the following.
Screening intervals. All patients who are placed on long-term HCQ treatment should have a baseline screening within the first year of beginning treatment. An initial fundus evaluation of the macula is critical to rule out preexisting disease that might make the retina more susceptible or screening difficult. Baseline visual fields (VFs) and spectral-domain optical coherence tomography (SD-OCT) scans are useful but not essential, unless abnormalities are present at baseline.
Initially, annual screening can be deferred, unless the patient is in a high-risk group. But beginning at the 5-year mark, all patients should be screened every year. And as the guidelines note, during each patient visit, the ophthalmologist should check the HCQ dosage relative to the patient’s weight and ask about any changes in systemic status, notably weight loss, kidney disease, and/or tamoxifen use.
Screening technology. Modern examination tools allow ophthalmologists to catch retinal damage at the earliest stage. Once regular screening for HCQ toxicity begins, the most important tests are SD-OCT and automated VFs.2 Additional tests include fundus autofluorescence (FAF), which can show damage topographically, and the multifocal electroretinogram (mfERG), which can provide corroboration for VFs.
“FAF is hard to interpret sometimes, but you can pick up a glow as toxicity develops,” Dr. Marmor said. “You hope to catch changes before you see any black areas.”
Screening Asian patients. Regarding VFs, “the 10-2 field is a very intense examination of the central degrees; that’s where damage occurs in most non-Asian patients,” Dr. Marmor said. “With Asians, damage may occur outside the range of a 10-2 field, so you should do both 10-2 and 24-2 fields.”
The problem with doing both 10-2 and 24-2 fields is that “they take time and are very fatiguing,” he acknowledged. “As a result, I do SITA Fast fields on my Asian patients, and doing both takes about the same time as one conventional 10-2. The pattern deviation plot is printed out, and I can see the areas that are relatively insensitive.”
Ultra-widefield imaging also holds promise for screening Asian patients, Dr. Marmor said. With regard to SD-OCT, Dr. Kim said, “In our series, we could detect all cases with pericentral or mixed parafoveal and pericentral types of HCQ retinopathy by eccentric 6-mm SD-OCT scans. I think SD-OCT scans with broad coverage are a good screening method for Asian patients.”
Not recommended. Photography and direct exams are not sensitive and thus are not recommended for annual screening, Dr. Marmor said. “You can’t see changes reliably or early enough.”
And a patient’s self-reported symptoms also cannot serve as a reliable guide to the extent of damage, Dr. Kim said. Even though 4 of the 9 patients with HCQ retinopathy in his study had advanced damage, “only 1 of the 9 patients complained of visual disturbances at the time of diagnosis.”
Summing Up
The main point of the revised guidelines is that “you want to get people on the right dose,” Dr. Marmor said. “You need to inform the rheumatologist—and you need to inform the patient as well.”
But he cautioned against abandoning HCQ altogether. It’s important to remember that HCQ is “a remarkably safe drug to use if the dose is correct and you’re screening properly,” he said. For many patients with SLE, RA, and other connective tissue diseases, “it’s much safer than steroids and immunosuppressives.”
___________________________
1 Sharma TS et al. J Am Heart Assoc. 2016:5(1):e002867. doi:10.1161/JAHA.115.002867.
2 Marmor MF et al. Ophthalmology. 2016;123(6):1386-1394.
3 Melles RB, Marmor MF. JAMA Ophthalmol. 2014;132(12):1453-1460.
4 Melles RB, Marmor MF. Ophthalmology. 2015;122(1):110-116.
5 Lee DH et al. Ophthalmology. 2015;122(6):1252-1256.
6 Eo DR et al. J Korean Med Sci. 2017;32(3):522-527.
___________________________
Dr. Braslow practices with the NorthShore University Health Care System north of Chicago and is a clinical educator at the University of Chicago’s Pritzker School of Medicine. Relevant financial disclosures: NorthShore University Health Care System: E.
Dr. Kim is associate professor of ophthalmology at Sungkyunkwan University School of Medicine’s Samsung Medical Center in Seoul and a visiting scholar at the Oregon Health & Science University’s Casey Eye Institute in Portland. Relevant financial disclosures: None.
Dr. Marmor is professor of ophthalmology at Stanford University and a retina specialist at Byers Eye Institute in Palo Alto, Calif. Relevant financial disclosures: None.
See the disclosure key at www.aao.org/eyenet/disclosures.
Excessive Dosing Still a Problem
Rebekah A. Braslow, MD, had been out of general ophthalmology practice for a few years before she moved to her current position north of Chicago. “I used my spare time to review some of the pertinent practice guidelines, including those on HCQ dosing. Once I started practicing, I realized that quite a few patients were overdosed.”
Initially, she thought that those patients were the exception. “However, after a few months, I saw a consistent pattern emerging, suggesting that the guidelines were not widely followed at our institution,” she said.
This prompted her to do a system-wide analysis on the entire patient population of her institution, using the electronic health record (EHR) system to identify and analyze patient data. The result: Of 554 patients on HCQ, some 50% had been placed on excess initial doses according to the 2011 guidelines, and 47% were on excess initial doses according to the 2016 guidelines.1
“Following the carefully conceived and validated HCQ dosing guidelines seemed like a very straightforward and natural strategy to keep our patients safe,” Dr. Braslow commented. “The fact that this was not done suggested a disconnect between our desire to protect our patients from medication toxicity and our day-to-day-practice.”
EHR to the rescue? Dr. Braslow came up with a potential solution: Use the same EHR system. “I thought it might be possible to translate the guidelines into a simple set of EHR alerts that would ‘take the remembering and thinking out of HCQ dosing’ to improve adherence, without requiring extra efforts from the prescribing physicians.”
Her idea has been well received, she said. “Once we had their attention, our rheumatology colleagues put together an HCQ task force of physicians and IT staff, with the goal of developing an easy-to-follow EHR alert that provides a guideline-compliant dose recommendation for each patient at the point of care.”
Unsurprisingly, there have been some EHR-related hurdles. “Tweaking the EHR seemed conceptually straightforward but proved surprisingly tricky to implement,” Dr. Braslow acknowledged. But a pilot program is now up and running, and the task force looks forward to implementing the final version this year.
___________________________
1 Braslow RA et al. Ophthalmology. 2017;124(5):604-608.
|