By Rebecca Taylor, Contributing Writer, interviewing Chiu M. Gemmy Cheung, MBBS, FRCOphth, Gregg T. Kokame, MD, MMM, and Timothy Y.Y. Lai, MD, FRCOphth
Download PDF
Is polypoidal choroidal vasculopathy (PCV) on your radar these days? If not, it should be. Recent studies have revealed a concerning picture of underdiagnosis of PCV, especially among ethnic groups previously thought to be relatively unaffected.1
Risk of blindness. “We’ve learned a lot about PCV over the past 10 years,” said Gregg T. Kokame, MD, MMM, at the University of Hawaii in Honolulu.
We now know that PCV is a subtype of neovascular age-related macular degeneration (AMD)—and that, if left untreated, it can rapidly progress to blindness or hemorrhage. “Just a bit of gradual leakage in the macula with some recurrent episodes of bleeding can cause visual problems, and breakthrough hemorrhage and hemorrhagic retinal detachment” have been reported with the condition, said Timothy Y.Y. Lai, MD, FRCOphth, at the Chinese University of Hong Kong.
Role in anti-VEGF resistance. Research into the condition’s prevalence has also shed light on its role in anti-VEGF resistance. “In all ethnic groups, PCV predicts anti-VEGF resistance, so it’s critical to make the diagnosis,” Dr. Kokame said.
Diagnostic Challenge
“Diagnosis of PCV has always posed a challenge because indocyanine green angiography [ICGA] has been the gold standard for diagnosis,” said Chiu M. Gemmy Cheung, MBBS, FRCOphth, at Duke-NUS Medical School in Singapore. “But ICGA is invasive and expensive, and it requires special equipment.”
The initial studies of PCV “were done using fundus camera ICG, which is much less sensitive than the scanning laser ophthalmoscope [SLO] we now use with ICGA, so initial evaluations of prevalence were very low,” said Dr. Kokame.
Rethinking ethnicity. Until relatively recently, the presumption was that PCV primarily affects those of Asian ancestry. But in a study among patients with wet AMD, Dr. Kokame found PCV in 51.6% of Asians, 28.6% of Pacific Islanders, and 31.9% of Caucasians—a rate much higher for Whites than previously thought.2
This finding is bolstered by other research, Dr. Kokame said: “A number of papers from Canada and Europe show that PCV is underdiagnosed in Caucasians—and that 20% to 30% of Caucasian patients initially diagnosed with exudative AMD actually have PCV.”
The condition is not as underdiagnosed in Black patients, he said, “because they often present with larger abnormal vessels and significant bleeding, visible on a fundus exam.”
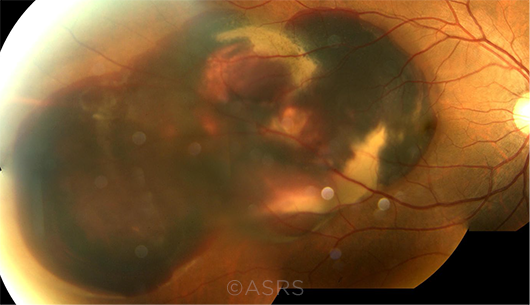 |
|
BLEEDING RISK. PCV can cause subretinal hemorrhage, as seen here in this fundus photo of a 52-year-old man. This image was originally published in the ASRS Retina Image Bank. Young-Gyun Kim, MD. Polypoidal Choroidal Vasculopathy With Massive Subretinal Hemorrhage. Retina Image Bank. 2013; Image Number 5099. © The American Society of Retina Specialists.
|
Coming to a Consensus
The problem of underdiagnosis spurred the Asia Pacific Ocular Imaging Society (APOIS) workgroup on PCV to tackle two challenges: Defining diagnostic criteria3 and comparing the diagnostic sensitivity and specificity of less-expensive OCT to ICGA.4
Their goal? A more accessible, affordable PCV diagnosis worldwide.
Defining terms. The APOIS recommended the following consensus terminology:
The polypoidal lesion. It may be tempting to shorten polypoidal lesion to polyp, said Dr. Cheung, “but since we can see a lumen and see dye filling it, we recommend the term polypoidal lesion since it appears to be more of a vascular lumen than a solid lump.”
The branching neovascular network. The vascular network has been rebranded as neovascular. “The depth of the lesion is generally agreed to have breached Bruch membrane—exactly where we would find a type 1 neovascular membrane—and its exudation often responds to anti-VEGF, so we recommend the term branching neovascular network,” said Dr. Cheung.
With PCV, said Dr. Kokame, “The blood vessels develop a bulge at the end of the vessels in the branching neovascular network—or, sometimes, within them. You see this beautiful network and then several of these aneurysmal dilations or polypoidal lesions.”
Identifying key diagnostic criteria. “We initially evaluated the performance of nine signs for differentiating PCV from neovascular AMD,” said Dr. Cheung. “It’s now down to three primary criteria that can be detected without needing ICGA, and we can differentiate PCV from typical neovascular AMD with about 90% accuracy.”
The APOIS proposed the following signs to differentiate PCV from typical neovascular AMD.
Using OCT. The three primary signs seen on OCT are:
- a sharp-peaked pigment epithelial detachment (PED), which appears as an inverted U or thumb-like protrusion;
- a ring-like lesion under the retinal pigment epithelium (RPE), possibly with a hyperreflective center; and
- a complex-shaped RPE elevation, with a hyperreflective branching neovascular network.
The first two signs can be seen on OCT-B scans; the third can be visualized with en face OCT.
Additional signs that can be observed on OCT are:
- a complex or multilobular PED;
- a double-layer sign (the split between the RPE and Bruch membrane, often where the branching neovascular network component lies);
- thick choroid with dilated Haller layer vessels; and
- fluid compartment with predominant subretinal fluid.
Using color fundus photography. Clinicians should look for an orange nodule appearing as a subretinal round elevation.
With regard to this sign, Dr. Cheung said, “If patients still have persistent fluid after three loading doses of anti-VEGF, we again look for the sharp-peaked PED and the sub-RPE ring—but now the third sign is an orange nodule, which may be due to the resolution of subretinal fluid and hemorrhage after initial treatment.
In addition, extensive subretinal hemorrhage may be seen.
Validating the role of OCT. In the second report, Dr. Lai noted, “We used only OCT to determine the PCV spot size—and by looking at the OCT findings alone, we could treat 100% of the polypoidal lesions and about 90% of the branching neovascular network.”
The significance of the orange nodule was identified in this report, particularly in anti-VEGF nonresponders.4 The nodules “can often be seen more clearly once fluid and blood decrease after initial anti-VEGF treatment,” the authors wrote.
Other studies have validated the APOIS findings; a 2021 study in Singapore used OCT and fundus photography to identify asymptomatic PCV in a cohort of 961 ethnic Chinese without using ICGA.5
PCV Features in Asians and Caucasians
Corvi et al. compared potential PCV features in 128 Asians and 122 Caucasians using multimodal imaging (color fundus photography, spectral-domain OCT, fluorescein angiography, and ICGA). All were treatment-naive.
|
FEATURE
|
ASIAN
|
CAUCASIAN
|
|
Subretinal hemorrhage
|
53.9%
|
24.6%
|
|
Pachyvessels
|
84.4%
|
28.7%
|
|
Choroidal vascular hyperpermeability
|
70.3%
|
17.2%
|
|
Widespread polypoidal lesions
|
19.5%
|
8.2%
|
|
Drusen
|
49.2%
|
79.5%
|
|
BCVA
|
0.7 logMAR
|
0.4 logMAR
|
|
Size of hemorrhage
|
7.5 ± 15.2 mm2
|
1.3 ± 3.3 mm2
|
|
BCVA = best-corrected visual acuity
SOURCE: Corvi F et al. Am J Ophthalmol. Published online Aug. 23, 2021.
|
Additional OCT Nuances
OCT B-scans. In his research, Dr. Kokame found that OCT B-scans could be successfully used to diagnose PCV. The presence of characteristic inverted U-shaped elevations in the RPE were visible on the B-scans and helped differentiate PCV from typical wet AMD, he said. (See “OCT B-Scans Pin Down Dx of PCV,” News in Review, August.)
“PCV is actually a type 1 choroidal neovascularization growing between Bruch membrane and the RPE, but with aneurysmal dilations that cause the inverted U-shaped elevation,” said Dr. Kokame.
The condition “often presents just like typical exudative macular degeneration, with leaking and bleeding in the macula and significant vision loss. However, the characteristic finding of an inverted U-shaped lesion with heterogeneous reflectivity is seen in up to 57% of cases of PCV and not in typical exudative AMD,” he added.
It’s critical to review OCT B-scans before treatment, he noted. “After anti-VEGF, the ability to identify those lesions with OCT-B scan goes down to 27%.”
Are there other tips to differentiate PCV from typical exudative AMD? Other distinguishing features include greater height of subretinal fluid, more serous retinal detachment, more frequent RPE detachment, and more frequent subretinal hemorrhage, Dr. Kokame noted.
OCTA. With OCT angiography (OCTA), “you can look at the flow inside these lesions without using dye and see some of the branching neovascular network very well,” said Dr. Lai. “But sometimes the polypoidal lesions don’t show up well because of turbulent or slow flow inside them.”
Currently, swept-source and widefield OCTA, which give better resolution and depth-of-field analysis, are available primarily at research centers, said Dr. Lai. “But the price is going down; they’ll likely be commercially launched in the next few years.”
En face OCT. With regard to using en face OCT, “We found that the RPE-RPE fit slab is the most accurate in identifying PCV,” said Dr. Kokame. “The ORCC slab, from the outer retina to the choriocapillaris, is also useful. You can simply go back to an old OCT to select both of those.”
Sight-Saving Patient Education
How can clinicians educate patients about PCV? “Traditionally, we ask patients to look for scotoma, distortion, or waviness,” said Dr. Cheung. In addition, the condition may not be picked up if only one eye is unaffected, she said. “One trick is to advise patients to check their eyes individually: Cover one eye at a time and look at a straight-edged object like a window or doorframe.”
Dr. Cheung noted that phone-based apps are being developed, with the goal of furthering early detection.
But at this point, multimodal imaging and color fundus photography have a key role to play in making the diagnosis, particularly in those settings where ICGA is either unavailable or not routinely used.
The bottom line: While PCV presents similarly to wet AMD, an early differential diagnosis can save vision. With PCV, “there’s leaking under the retina, macular edema, intraretinal edema, subretinal hemorrhage, and detachment, so it looks like typical exudative AMD until you do more specific testing,” said Dr. Kokame.
___________________________
NEXT MONTH: Part 2: treatment.
___________________________
1 Kokame GT et al. Ophthalmol Retina. 2021;5(10):954-961.
2 Kokame GT et al. Ophthalmol Retina. 2019;3(9):744-752.
3 Cheung CMG et al. Ophthalmology. 2021;128(3):443-452.
4 Teo KYC et al. Ophthalmol Retina. 2021;5(10):945-953.
5 Fenner BJ et al. Ophthalmol Retina. Published online Sept. 21, 2021.
___________________________
Dr. Cheung is professor of ophthalmology and visual sciences at Duke-NUS Medical School in Singapore and head and senior consultant of the Medical Retina Department at the Singapore National Eye Centre. Relevant financial disclosures: Allergan: C,L,S; Avirmax: C,L,S; Bayer: C,L,S; Boeringher Ingelheim: C,L,S; Carl Zeiss: C,L,S; Novartis: C,L,S; Roche: C,L,S; Topcon: C,L,S.
Dr. Kokame is chief of ophthalmology and clinical professor at the University of Hawaii School of Medicine and founding partner, senior consultant, and CEO of Retina Consultants of Hawaii, both in Honolulu. Relevant financial disclosures: Bausch + Lomb: C,L; Carl Zeiss: L; Genentech: S; Novartis: S; Regeneron: S; RegenxBio: S.
Dr. Lai is a clinical professor (honorary) of ophthalmology and visual sciences at the Chinese University of Hong Kong. Relevant financial disclosures: Allergan: C,L,S; Bayer: C,L,S; Boeringher Ingelheim: C,L,S; Novartis: C,L,S; Roche: C,L,S.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Cheung Allergan: C,L,S; Avirmax: C,L,S; Bayer: C,L,S; Boeringher Ingelheim: C,L,S; Carl Zeiss: C,L,S; Novartis: C,L,S; Roche: C,L,S; Topcon: C,L,S.
Dr. Kokame Bausch + Lomb: C,L; Bayer: C; Carl Zeiss: L; Genentech: S; Iveric: S; Novartis: S; Regeneron: S; Roche: C; RegnxBio: S; Salutaris: S.
Dr. Lai Allergan: C,L,S; Bayer: C,L,S; Boeringher Ingelheim: C,L,S; Novartis: C,L,S; Roche: C,L,S.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|