Download PDF
This month, News in Review highlights selected papers from the original papers sessions at AAO 2021. Each was chosen by the session chairs because it presents important news or illustrates a trend in the field. Only four subspecialties are included here; papers sessions also will be held in five other fields. For up-to-date information, check the Mobile Meeting Guide (aao.org/mobile).
There's more good news on the Port Delivery System (PDS), which has the potential to reduce the burden of anti-VEGF treatment for patients with neovascular age-related macular degeneration (AMD). In an experimental study, the implant was found to effectively deliver ranibizumab over a six-month period.
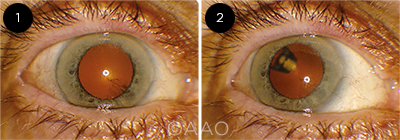 |
IN PLACE. A patient with the PDS in place. The implant cannot be seen when the eye is in the primary position (1) but is visible when the patient looks up (2). Adapted from: Campochiaro PA et al. The Port Delivery System with ranibizumab for neovascular age-related macular degeneration. Ophthalmology. 2019;126(8):1141-1154. This is an open access article under the CC-BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.00).
|
PDS specifics. The PDS (Roche) is a permanent refillable implant roughly the size of a small pellet. It is surgically implanted into the vitreous and continuously delivers a customized formulation of ranibizumab over a period of months. In preliminary results from the phase 3 Archway study, 98.4% of those who received the PDS did not require supplemental treatment before the first refill-exchange at 24 weeks.1
Study specifics. For this in vitro study, implants were filled with ranibizumab 10, 40, or 100 mg/mL. Drug concentrations were measured via automated sample preparation, and concentration data were used to calculate a mean daily ranibizumab release rate during an initial fill and at three refill exchanges.
Main outcomes included the release rate of ranibizumab, the active release rate (measured in grams per day), and the amount of drug retained in the implant at the end of the study.
Drug release. Approximately 70% of ranibizumab 100 mg/mL was released from the implant during the six months, said Mark R. Wieland, MD, at Northern California Retina Vitreous Associates in San Jose.
The mean active release rate was 3.95 g/day at the initial fill and 3.99, 3.85, and 4.0 g/day at the first, second, and third drug refill exchanges, respectively. Changing the initial concentration of ranibizumab from 10 to 100 mg/mL increased the initial drug release rates from 2 to 17 g/day.
Drug retention. During refill-exchange, approximately 98% of the previous implant contents were replaced with fresh drug during one 100-mg/mL refill.
Conclusion and next steps. The results indicate that the PDS can continuously and reproducibly deliver ranibizumab over a period of months while maintaining potency, Dr. Wieland said.
Next up: regulatory review. This summer, Roche submitted an application to the FDA. The implant is also under review by the European Medicines Agency.
—Jean Shaw
|
The Port Delivery System With Ranibizumab: A New Paradigm for Long-Acting Retinal Drug Delivery. Presented during the third retina original papers session. When: Monday, Nov. 15, at 11:54 a.m. Where: Room 255-257.
|
___________________________
1 Awh CC et al. Primary analysis results of the phase 3 Archway trial of the Port Delivery System with ranibizumab for patients with neovascular AMD. Paper presented at: Retina Society 2020 Virtual; July 26, 2020.
___________________________
Relevant financial disclosures—Dr. Gedde: None.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Chang AcuFocus: S; Allergan: C,L,S; Carl Zeiss Meditec: C,L,S; Johnson & Johnson Vision: C,L,S; Omega Ophthalmics: C,O.
Dr. Gedde None.
Dr. Uddaraju None.
Dr. Wieland Roche: C.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|
More from this month’s News in Review