By Keng Jin Lee, PhD, and Aliyah Kovner, MSC, Academy Online Medical Writers, interviewing Jason Hsu, MD, Demetrios G. Vavvas, MD, PhD, and David N. Zacks, MD, PhD
Download PDF
Detachment of the neurosensory retina from the retinal pigment epithelium (RPE) can occur after trauma or as the result of disease. In either case, it requires intervention soon after detachment. Rhegmatogenous and tractional detachments must be reattached surgically; and current techniques, including scleral buckle, pneumatic retinopexy, and vitrectomy, have anatomic success rates reported to be as high as 80% to 96%.1 Yet, despite advances in retinal detachment surgery, many patients are left with poor visual outcomes as a result of irreparable damage to photoreceptor cells.
Photoreceptor Cell Death
During the perioperative period, death of photoreceptor cells leads to permanent visual loss, particularly when the macula is involved. It is estimated that only 40% of patients with macula-off rhegmatogenous detachments recover visual acuity of 20/40 or better.2 In addition, the presence of proliferative vitreoretinopathy puts a patient at high risk of subsequent re-detachments due to contraction of scar tissue. These repeated episodes of tissue layer separation also lead, ultimately, to photoreceptor death.
“The problem is, you can have an anatomic success and a functional failure, which is kind of the joke about success in retinal detachment,” said David N. Zacks, MD, PhD, at the University of Michigan in Ann Arbor. “Especially when the detachment affects the macula. You can potentially lose a lot of vision, and that visual loss is fundamentally due to the death of the photoreceptor cells.”
To mitigate these complications, researchers are investigating the pathways that inhibit apoptotic and nonapoptotic forms of cell death. And they have identified several pharmacologic agents that show potential to improve visual and anatomic outcomes when used as adjunct therapies alongside surgery.
 |
|
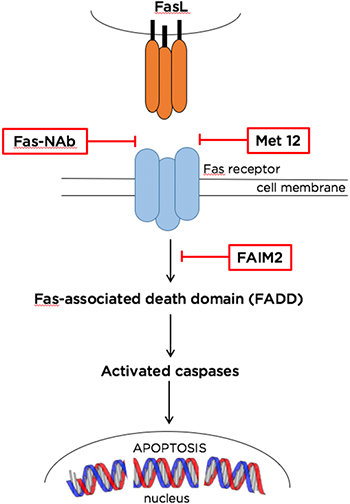
FAS-MEDIATED APOPTOSIS PATHWAY. Inhibition of photoreceptor cell death using experimental pharmacotherapies can be achieved by targeting various signaling proteins in the Fas pathway. The agents shown here work upstream or downstream of Fas, blocking the activation of caspases and apoptotic cell death. Abbreviations: FAIM2, Fas apoptotic inhibitory molecule 2; FasL, Fas ligand; Fas-NAb, Fas-receptor- neutralizing antibody; Met 12, Met YLGA 12-mer (YLGA-amino acid sequence motif containing tyrosine, leucine, glycine, and alanine).
|
Apoptosis Pathways
In the United States, surgery is typically scheduled several days to 1 week after the detachment has occurred. Despite this rapid response, a significant number of photoreceptor cells may die within the first few days while the tissue remains physically separated from the underlying RPE and choroidal vessels. When a photoreceptor cell experiences this type of trauma, multiple signaling pathways are activated to induce programmed, extrinsic cell death—largely by apoptosis but also through the mechanisms of necrosis and autophagy.
Role of caspase. Apoptosis is an orderly cell death pathway involving the activation of caspases—a family of proteases that degrade more than 600 cellular components. At the end of the signaling cascade, “executioner” caspases dissolve the nuclear envelope, collapse the cytoskeleton, and tag the phospholipid membrane enclosing the remaining contents (by this point reduced to a bleb) for phagocytic attack and removal.
The Fas pathway. Many apoptotic pathways have been elucidated, but the most promising target for inhibition is the death receptor Fas. A member of the large cell death–signaling tumor necrosis factor (TNF) family, the Fas receptor is a transmembrane protein that—when bound—forms a signaling complex inducing apoptosis. An experimental model of retinal detachment has shown that separation of the retina from the RPE induces transcription of genes for the Fas receptor.3 The majority of cell death, at least in the acute phase, appears to be mediated through Fas receptor activation, said Dr. Zacks.
Two Fas discoveries. Early investigations from Dr. Zacks’ lab into inhibiting this pathway yielded 2 experimental molecules: Fas-receptor-neutralizing antibody (Fas-NAb) and a small inhibitory RNA (siFas) that prevents Fas mRNA translation. Both methods were shown to significantly reduce photoreceptor death in rats and to maintain higher cell counts and outer nuclear layer (ONL) thickness for up to 2 months after an induced separation.4
Fas-NAb. To quantify apoptosis and cell death, Dr. Zacks and colleagues employed TUNEL assays. This is a widely used histological method that stains cells containing DNA cut by death enzymes. They found that compared with controls, rat eyes injected with Fas-NAb were 60% less likely to undergo the apoptotic cascade on day 3 post-separation (the peak time point for cell death in rats). And 2 months after the separation, eyes treated with a single Fas-NAb injection showed significantly better preservation of ONL thickness.4
siFas. Dr. Zacks’ work on siFas demonstrated similar effectiveness against photoreceptor cell death with significant increases, compared with controls, in the optic nerve cell count (68%) and thickness (73%) at 2 months post-separation.4
Therapies on the Bench
The success of this initial work has paved the way for several new potential therapies that also exploit the utility of the Fas receptor.
Targeting the Fas ligand–receptor complex. Fas-triggered caspase activation begins with the Fas receptor binding to the Fas ligand (FasL). FasL comes in 2 forms: soluble and membrane-bound, said Demetrios G. Vavvas, MD, PhD, at Massachusetts Eye and Ear in Boston. Although both forms can bind to the Fas receptor, only the membrane-bound form triggers apoptosis. Subretinal injections of recombinant soluble FasL during induction of retinal detachment in membrane-bound FasL transgenic mice reduced photoreceptor cell death by approximately 50%.5
This work is supported in a separate study by Dr. Zacks’ lab, which is investigating a small peptide inhibitor called Met 12 to block FasL receptor binding. Initial in vitro studies showed positive results, with Met 12 demonstrating dose-dependent inhibition of caspase 8 activation in a photoreceptor cell line.6 The molecule also decreased caspase 3, 8, and 9 activity by about 50% in rats with induced retinal detachment, compared to controls. This corresponded to an approximately 77% drop in TUNEL-positive photoreceptors 3 days after retina/RPE separation.6
Fas-mediated ERK pathway. Fas apoptotic inhibitory molecule 2 (FAIM2) belongs to the Lifeguard family of anti-apoptotic proteins that prevent cell death by blocking formation of the Fas-associated death domain, which activates caspase molecules. Interestingly, expression of both Fas intermediates and FAIM2 increases in the retina after a detachment occurs. One study showed that increases—thought to be mediated by ERK stress kinase signaling7—began in as little as 4 hours after an induced separation both in vivo and in vitro. Furthermore, siRNA knockdown of FAIM2 led to earlier and more intense photoreceptor apoptosis, suggesting that FAIM2 serves as a neuroprotectant that delays cell death after Fas activation.7
Modulation of FAIM2 expression represents a potentially novel therapeutic approach that utilizes one of the body’s innate antideath autoregulation pathways, but it is still in early stages of testing with in vivo models.
Beyond apoptosis. Although apoptosis prevention remains the most active area of interest in retinal neuroprotection, a number of recent studies have investigated the roles of autophagy and necrosis. These processes, which are regulated by specific proteins, may also significantly contribute to photoreceptor cell loss. Thus, it is plausible that combined targeting of these different modes of cell death could provide an effective and more comprehensive neuroprotection strategy.
Necrosis-mediated cell death. While a plethora of research has shown that caspase signaling is activated following retinal detachment, recent studies have shown that the pan-caspase inhibitor Z-VAD decreased apoptosis in a rodent model of retinal detachment, but it exacerbated necrosis.8 Scientists also found that the receptor interacting protein (RIP) kinase-mediated necrosis was upregulated when caspase pathways are blocked, suggesting a compensating mechanism for apoptosis inhibition. Interestingly, RIP kinase is also Fas mediated and forms a death-signaling complex after Fas activation. RIP kinases are hypothesized to increase reactive oxygen species (ROS) production, which leads to necrosis. Furthermore, Trichonas et al. showed that cotreatment with both Z-VAD and necrostatin-1, a small-molecule inhibitor of RIP kinase, substantially reduced photoreceptor loss.8
The Path Ahead
The field of adjunct therapies is exciting to many retina specialists. Yet, at the same time, the status of the research may seem disheartening to some specialists. There are many promising leads, such as Met 12 or FAIM2, yet none have reached a phase 1 trial. “It’s always great to hear about theoretical therapies—but then frustrating to learn that they are far from proven to be of benefit in human trials,” said Jason Hsu, MD, at Wills Eye Hospital in Philadelphia.
While Dr. Vavvas does not think that any individual therapy can prevent all cell death, inhibiting Fas itself could be an approach to target multiple pathways. “The data on Fas are quite strong,” he said. “If I were to put my bet on only one approach, that is the first one that I would try.”
Critical window. Drug candidates will have to undergo multicenter collaborative studies to continue their development and define their role in optimizing outcomes during management of retinal detachment. However, this type of study presents logistical difficulties, particularly because of the brief time window between diagnosis and surgical repair. Investigators would need to step in to assign either an active or placebo agent almost immediately.
Possible clinical testing. Despite these obstacles, Dr. Vavvas believes that the requirements of a prospective randomized trial could be met within a clinical setting. An experimental neuroprotective therapy could be administered immediately after diagnosis by intravitreal injection and could preserve photoreceptors while the patient awaits surgery.
Of a Fas phase 1 trial, which could take place within a year, he said,“There is quite a bit of interest among retinal surgeons to test these things, because it doesn’t inconvenience people. You just inject them at the time of diagnosis—it takes 5 minutes. Everyone is familiar with doing intravitreal injections now. And it doesn’t significantly change the protocol of what they have to do or the flow of their practice.”
Dr. Vavvas added that although some physicians may be concerned with logistics, many are eager to enroll patients and become involved. They are motivated by the hope of improving outcomes by increasing photoreceptor survival during the period between presentation and repair.
A starting cohort. After initial human safety and tolerability trials, Drs. Zacks and Vavvas both recommend a randomized controlled trial of patients with macula-off retinal detachment. This cohort represents an ideal starting point, as they are at most risk for poor outcomes. The trial itself could have a simple design: Everyone would get an injection prior to surgery—with active agent or sham—and visual outcomes could be compared.
Beyond Retinal Detachment
Dr. Vavvas sees a wide variety of applications for this therapy beyond retinal detachment. Retinal cell death remains the underlying cause of vision loss in many retinal conditions, including age-related macular degeneration, diabetic retinopathy, and retinitis pigmentosa. “This work would be proof of concept. If successful, this could see progress outside of ophthalmology, possibly even in neurological diseases,” he added.
Surgeons have come a long way in treating retinal detachment. And although they have good success in anatomic repair, the next step is to address the unmet need of preserving vision to the highest possible level by preventing photoreceptor cell death. Adjunct pharmacotherapies may be the bright spot on the horizon, but a clinical trial within the next year or two is necessary to substantiate their promise.
“The cavalry is on its way, we hope,” said Dr. Zacks with a laugh.
___________________________
1 Wubben TJ. Ophthalmology. 2016;123(7):1553-1562.
2 Campo RV et al. Ophthalmology. 1999;106(9):1811-1815.
3 Zacks DN et al. Invest Ophthalmol Vis Sci. 2004;45(12):4563-4569.
4 Zacks DN et al. Arch Ophthalmol. 2007;125(10):1389-1395.
5 Matsumoto H et al. Cell Death Dis. 2015;19(6):e1986.
6 Besirli CG et al. Invest Ophthalmol Vis Sci. 2010;51(4):2177-2184.
7 Bersirli CG. PLoS ONE. 2012;7(9):e46664.
8 Trichonas et al. Proc Natl Acad Sci. 2010;107(50):21695-21700.
___________________________
Dr. Hsu is assistant director of retina research at the Retina Service of Wills Eye Hospital and managing partner at Mid Atlantic Retina. Relevant financial disclosures: None.
Dr. Vavvas is codirector of the Ocular Regenerative Medical Institute, a clinician-scientist in the retina service of Massachusetts Eye and Ear Institute, associate professor of ophthalmology at Harvard Medical School, a principal investigator in the Angiogenesis Laboratory, and the incumbent of the Monte J. Wallace Ophthalmology Chair in Retina. Relevant financial disclosures: None.
Dr. Zacks is professor of ophthalmology and visual sciences the University of Michigan, Ann Arbor. Relevant financial disclosures: None.
For full disclosures and the disclosure key, see below.