Download PDF
Scientists have developed a gene therapy for the most common form of autosomal-dominant retinitis pigmentosa (RP) caused by mutations in the rhodopsin (RHO) gene—and successfully demonstrated that it can prevent retinal degeneration in a canine model, an approach that someday could be harnessed to halt the disease in humans.
The researchers designed an adeno-associated viral vector to knock down the expression of existing RHO (both normal and mutant genes) and replace them with a normal copy of human RHO.1 Retinal imaging and electroretinography showed that this approach kept the rod photoreceptors healthy and prevented retinal degeneration for at least 8 months of follow-up.
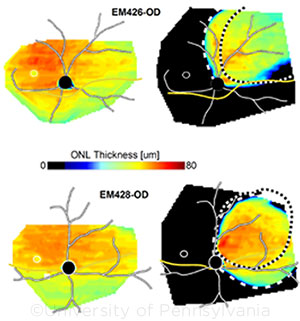 |
NOVEL STRATEGY. Topographical maps of outer nuclear layer thickness show how the combined RHO knockdown and replacement therapy protected against severe retinal degeneration in a naturally occurring canine model of autosomal-dominant RP (right panels).
|
A dual-purpose approach. This strategy differs in key ways from the gene augmentation approach that led to a commercially available gene therapy (Luxturna) for Leber congenital amaurosis (LCA). Because LCA is autosomal recessive, that viral vector was engineered solely to transduce the retinal pigment epithelium with a single copy of the RPE65 gene to produce the missing protein.
In autosomal-dominant RP, rod photoreceptor cells express rhodopsin proteins from both normal and mutant RHO. “The mutant protein produced in the rods either interferes negatively with the wild-type protein that comes from the normal allele, or it has on its own a toxic gain of function, meaning that this protein may be toxic to the cell and kill it over time,” said William A. Beltran, DVM, PhD, at the University of Pennsylvania in Philadelphia.
The scientists concluded that their treatment would have to not only prevent the mutant gene already there from producing abnormal, toxic rhodopsin but also provide sufficient normal rhodopsin protein for the rods to survive and function normally. But in order to do this, the therapy also would need to work against the more than 150 gene mutations known to cause autosomal-dominant RP. “The challenge was to develop a treatment that can address any mutation,” Dr. Beltran said.
Their solution was a dual-purpose viral vector: One part was targeted at “knocking down” all endogenous rhodopsin mRNAs, both mutant and normal, through a technique known as RNA interference. The second part consisted of normal human RHO that was modified to avoid degradation by the knockdown component.
“The rods would not be able to function, or survive over the long term, if you didn’t bring back a normal wild-type copy of RHO. The vector also delivers a gene that has been modified at some very specific sites so that it still produces the same amino acid sequence as the rhodopsin protein, but it is not knocked down at the RNA level,” said coauthor Alfred S. Lewin, PhD, at the University of Florida in Gainesville.
What’s next. The researchers will study whether the viral vector can successfully treat areas where the retina is already degenerating. “We’re going to be looking at whether we can intervene at stages that are clinically relevant—and target areas where there are still rod photoreceptor cells left that can be rescued,” Dr. Beltran said. “If we’re able to show that, that will expand the potential candidates for gene therapy.”
—Linda Roach
___________________________
1 Cideciyan AV et al. Proc Natl Acad Sci U S A. 2018;115(36):E8547-E8556.
___________________________
Relevant financial disclosures—Dr. Beltran: Foundation Fighting Blindness: S; NIH/NEI: S; Research to Prevent Blindness: S; The Shaler Richardson Professorship Endowmment: S; University of Pennsylvania: P. Dr. Lewin: NEI: S; University of Florida: P.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Beltran Foundation Fighting Blindness: S; NIH/NEI: S; Research to Prevent Blindness: S; The Shaler Richardson Professorship Endowmment: S; University of Pennsylvania: P. Dr. Lewin: NEI: S; University of Florida: P.
Dr. Grand None.
Dr. Grönlund Agreement Concerning the Research and Education of Doctors (Gothenburg): S; Bayer: L; Cronqvists Foundation: S; De Blindas Vänner: S; Gothenburg Medical Society: S; Swedish Society of Medicine: S.
Dr. Hsu Roche/Genentech: S; Ophthotech: C,S; Santen: S.
Dr. Lewis NEI: S; University of Florida: P.
Dr. Obeid None.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|
More from this month’s News in Review