By Mashal Akhter, MD, Michelle W. Latting, MD, and Adrienne W. Scott, MD
Edited By: Sharon Fekrat, MD, and Ingrid U. Scott, MD, MPH
Download PDF
Proliferative sickle cell retinopathy (PSR) is a vision-threatening complication of sickle cell disease (SCD). Ischemic events in the retina stimulate angiogenesis, resulting in retinal neovascularization.
SCD is caused by a mutation in the HBB gene, which encodes hemoglobin beta. PSR affects up to 40% of heterozygous (HbSC) patients and 20% of homozygous (HbSS) patients.1 Although most patients remain visually asymptomatic, the incidence of visual loss among patients with HbSS and HbSC affected by PSR has been reported as 31 per 1,000 eyes compared with 1.4 per 1,000 in eyes with nonproliferative disease.2
Epidemiology
The highest frequency of genotypes associated with HbS is seen in populations of African descent; however, high rates of at-risk genotypes are also seen in people of Mediterranean, Caribbean, South and Central American, Arabic, and East Indian descent.3
Patients with the HbSS genotype typically experience greater systemic morbidity, whereas those with the heterozygous genotypes (HbSC and HbS-beta thalassemia) are more likely to manifest PSR. Roughly 32% of HbSC patients will have retinal neovascularization with vitreous hemorrhage compared with 6% of HbSS patients.4 PSR risk increases with age and is observed more commonly in males. In individuals with HbSC, the peak onset of retinopathy is between 15 and 24 years in men and between 20 and 39 years in women.5
Pathophysiology
Red blood cells in patients with SCD are less pliable than normal, resulting in microvascular occlusive events. Subsequent retinal capillary hypoperfusion or nonperfusion and defective oxygen transport lead to local ischemia, which stimulates production of proangiogenic growth factors, such as vascular endothelial growth factor (VEGF), leading to retinal neovascularization. Inflammatory mediators and intracellular adhesion molecules also play an important role in the pathophysiology of PSR.
Retinopathy in HbSC vs. HbSS patients. There are several theories as to why individuals with HbSC genotypes are more likely to develop PSR than those with HbSS. In HbSC, the blood has greater viscosity and higher hematocrit levels, which may lead to small-vessel occlusion in the retinal vasculature.6 Another theory is that the HbSC state may produce less severe retinal vaso-occlusion, resulting in low-grade chronic ischemia and release of proangiogenic growth factors, thereby creating a more favorable environment for neovascularization.6 In contrast, individuals with the HbSS phenotype may be less likely to generate an angiogenic response because their vascular occlusion is more complete, producing more profound infarction and leading to retinal necrosis.
Retinal Neovascularization
In PSR, the new vessels grow in a frondlike configuration, which has classically been described as resembling a sea fan (Fig. 1). As this sea-fan neovascularization grows along the retinal surface and onto the posterior cortical vitreous, vitreous traction on the neovascular channels may result in intermittent vitreous hemorrhage. Vitreous hemorrhage can also be caused by minor ocular trauma or contraction of vitreous bands from a previous hemorrhage.
Spontaneous regression of the sea-fan neovascularization may occur in up to 60% of patients and is thought to result from autoinfarction.7
Sight-threatening complications. In addition to vitreous hemorrhage and vitreoretinal traction, vision-threatening complications of neovascularization include:
- Traction retinal detachment
- Traction retinoschisis
- Macular pucker
- Macular hole
- Retinal break
- Rhegmatogenous or exudative retinal detachment
Course of sea-fan neovascularization. Progression of retinal neovascularization in PSR is variable; some neovascular fronds remain relatively small and flat for many years before autoinfarcting, while others enlarge rapidly and coalesce with neighboring lesions to form larger, elevated, circumferential neovascular complexes.
Estimating the age of a PSR lesion is difficult; the clinician is limited to an assessment of the size and degree of elevation above the retinal plane and a semiquantitative assessment of the amount of perfusion and leakage on fluorescein angiography. Disease severity may be quantified by the number of perfused and autoinfarcted sea fans, along with the circumferential extent of PSR.
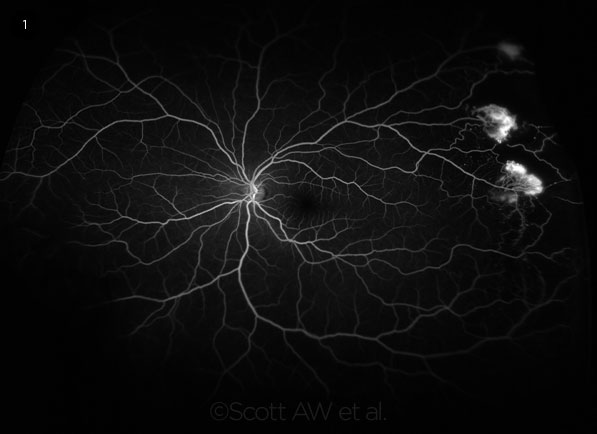 |
|
SEA FANS. Ultra-widefield fluorescein angiography demonstrates bright hyperfluorescence of sea-fan neovascularization caused by PSR.
|
Screening
Because PSR lesions are typically located in the retinal periphery, individuals with earlier stages of PSR often remain visually asymptomatic. It is recommended that patients with SCD have dilated funduscopic examinations every 1 to 2 years beginning at age 10,8 preferably by a retina specialist. (These recommendations are based on expert panel consensus, not on large data-driven studies.)
Imaging Modalities
Ultra-widefield imaging (up to 200-degree field of view) is useful in documenting and following PSR lesions. Ultra-widefield fundus photography (UWF-F) and ultra-widefield fluorescein angiography (UWF-FA) have been shown to detect a more-advanced stage of sickle cell retinopathy compared with fundus examination alone.9 UWF-FA is helpful in showing the extent of leakage from sea-fan lesions and, particularly, in documenting the extent and longitudinal progression of peripheral retinal ischemia (Fig. 1).
Macular thinning, which increases with age, is common across all genotypes in patients with sickle cell retinopathy.10 Diffuse thinning may be present throughout the macula, producing a blunted angle of foveal contour, called foveal splay, seen on spectral-domain optical coherence tomography (SD-OCT). Focal thinning may also occur in the temporal subfields, a known watershed zone for the macular vasculature (Fig. 2).11 A recent study showed that SD-OCT in conjunction with complete ophthalmologic examination may lead to earlier detection of retinopathy in children less than 10 years old.12
OCT angiography (OCTA) is an emerging imaging modality that can reveal abnormalities in the retinal microvasculature in both proliferative and nonproliferative sickle cell retinopathy. The macular vessel density, specifically in the deep retinal plexus, as seen on OCTA, has been shown to correlate with peripheral nonperfusion seen on UWF-FA in patients with HbSC disease (Fig. 3).13
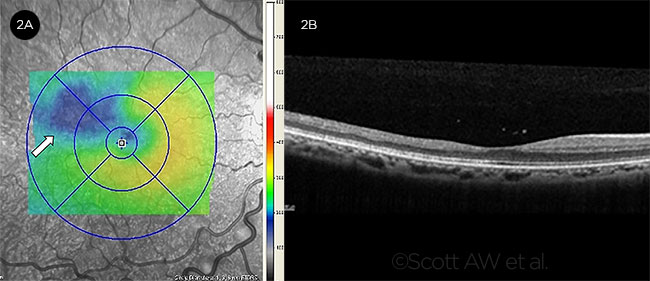 |
|
FOVEAL FINDINGS. (2A) SD-OCT demonstrates focal thinning (arrow) within the temporal foveal region in a patient with HbSS disease. (2B) Foveal splaying (saucerization of the foveal pit) is noted in this patient.
|
Treatment
Treatment protocols for PSR are not standardized; however, they share the common goal of reducing the risk of progression of neovascular lesions to vitreous hemorrhage and/or retinal detachment.4 In particular, the aims of therapy are to
- Render the lesion avascular and less likely to bleed,
- Reduce or arrest the chronic transudation of plasma into the vitreous, and
- Prevent or reduce the vitreoretinal traction that may result from progressive enlargement and forward growth of the lesion into the vitreous.
Treatments that increase fetal hemoglobin, or HbF, such as hydroxyurea, omega-3 fatty acids, and erythropoietin may help to decrease the morbidity of SCD by interfering with the polymerization of HbS and reducing the load of inflammatory mediators.4 However, it is not yet known how systemic sickle cell status or systemic treatments such as hydroxyurea or exchange transfusions (in which the patient’s blood is removed and replaced by a donor’s blood) may influence the progression of retinopathy or the development of PSR.
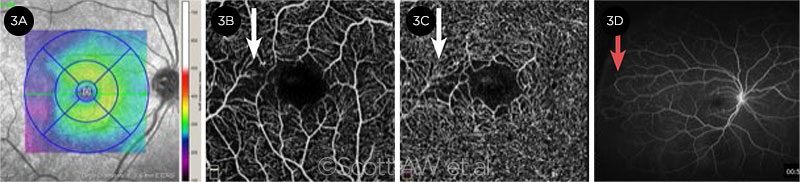 |
|
MULTIMODAL VIEWS. Right eye of HbSS patient shows vascular abnormalities in the macula and the retinal periphery. (3A) Infrared retinal SD-OCT image, with areas of retinal thinning (blue) noted in the outer temporal subfields. (3B) OCTA of the superficial and (3C) deep capillary plexuses with areas of abnormal vascular flow in the temporal juxtafoveal macula (arrows). (3D) UWF-FA shows vascular anastomotic loops in stage 2 PSR (arrow) and temporal nonperfusion in the retinal periphery.
|
Laser Photocoagulation
Although scatter laser photocoagulation is the current mainstay for managing PSR, there is no evidence-based, widely accepted treatment regimen. Moreover, the published literature is inconclusive on whether retinal laser photocoagulation is effective in changing visual outcomes in PSR.14
Patient selection. Laser photocoagulation is generally considered if there is rapid growth or elevation of neovascular lesions, progressive vitreous traction and/or bleeding of a neovascular lesion, or increase in the number of neovascular lesions. This treatment modality may also be considered in cases of extensive peripheral nonperfusion, as well as in patients with neovascularization who are likely to have poor adherence to follow-up schedules or those who have visual impairment or blindness in the fellow eye.
Technique. Laser photocoagulation may be performed as local focal treatment to barricade a sea-fan neovascular complex or in a 360-degree circumferential delivery to the peripheral retina (Fig. 4).
Emerging Therapies
Anti-VEGF agents. A single intravitreal injection of anti-VEGF medications such as bevacizumab or ranibizumab may lead to partial regression of sea-fan neovascularization and decreased leakage on fluorescein angiography.15 The optimal frequency of intravitreal anti-VEGF injections in PSR is unknown; however, evidence suggests that the degree of fibrosis of the sea-fan lesion in response to anti-VEGF therapy may help to determine the schedule of further injections.15
Anti-VEGF injection may also be used as an adjunctive therapy for patients with recurrent vitreous hemorrhage who have already received laser treatment. Similar to its use in selected cases of traction retinal detachment and proliferative diabetic retinopathy, bevacizumab has proved helpful in decreasing intraoperative bleeding and dissecting neovascular fronds from the retinal surface when injected prior to vitrectomy.
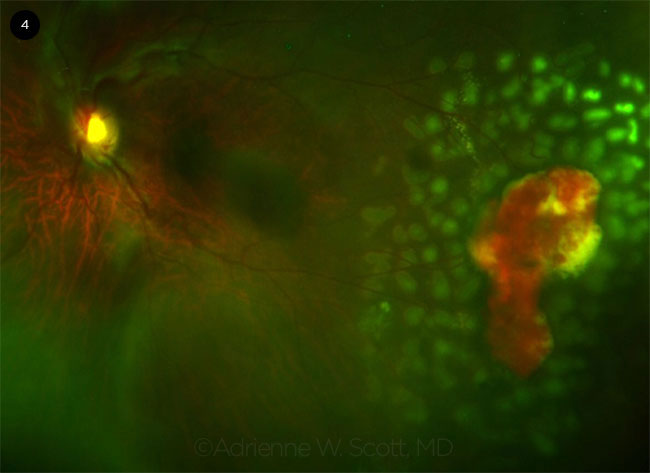 |
|
LASER EFFECTS. A sea-fan lesion with localized hemorrhage is depicted on UWF fundus photography immediately after scatter laser treatment in a patient with HbSC disease.
|
Surgical Options
Surgery for PSR may be indicated in eyes with retinal detachment, nonclearing vitreous hemorrhage, vitreous hemorrhage that occurs bilaterally or in a monocular patient, macular hole, or symptomatic vitreomacular traction. In all such patients, careful preoperative planning is required, incorporating a multidisciplinary approach with the participation of the vitreoretinal surgery, hematology, and anesthesiology teams.
Surgical considerations. General anesthesia is preferred over local, as it allows for optimal intraoperative pain control. If general anesthesia is contraindicated, sub-Tenon’s anesthesia is preferred over retrobulbar block, as even modest elevations in intraocular pressure (IOP) may compromise the ophthalmic circulation in sickle cell patients.
Preoperative partial-exchange blood transfusion to increase hemoglobin A to more than 60% should be considered. During surgery, it is important to ensure adequate intraoperative hydration, oxygenation, and careful attention to IOP control. Postoperative exchange transfusion and hospitalization for maximal oxygenation, hydration, and pain management should be considered in patients with SCD.
Vitrectomy. In selected cases of PSR, vitrectomy is indicated in order to achieve clearing of vitreous hemorrhage or to facilitate retinal reattachment.
Retinal traction from neovascular complexes must be relieved. Use of a segmentation technique allows for vertical cutting of the fibrovascular tissue into small sections to relieve circumferential traction. In contrast, delamination involves removing the membranes through horizontal dissection in the plane between the retina and the fibrotic tissue and may be more likely to cause iatrogenic retinal breaks. Sea-fan neovascular complexes are located in the anterior retina, where the tissue is more ischemic and thin and, thus, prone to iatrogenic breaks with even minimal manipulation. Special attention must be paid to management of IOP during vitrectomy, as patients with sickle trait are more prone to vascular occlusions with even modest and transient IOP elevations.
Scleral buckle. Historically, encircling buckles have been relatively contraindicated in patients with SCD, as scleral buckles have been shown to decrease retinal blood flow and alter perfusion of the anterior choroid, ciliary body, and ciliary processes. However, modern, thinner scleral buckles can be used safely in eyes with PSR and may be helpful in supporting areas of peripheral retinal pathology. To avoid anterior segment ischemia in patients with sickle cell status, the surgeon should take care not to place a scleral buckle too tightly.
___________________________
1 Ahn ES et al. Posterior pole manifestations of hematologic diseases. In: Arevalo JF, ed. Retinal and Choroidal Manifestations of Selected Systemic Diseases. New York: Springer Science Business Media; 2013.
2 Moriarty BJ et al. Eye (Lond). 1988;2(Pt 3):330-335.
3 Elagouz M et al. Surv Ophthalmol. 2010;55(4):349-377.
4 Fox PD et al. Br J Ophthalmol. 1990;74(3):172-176.
5 Ballas SK et al. Scientific World Journal. 2012;2012:949535. doi:10.1100/2012/949535.
6 Fadugbagbe AO et al. Ann Trop Paediatr. 2010;30(1):19-26.
7 Lutty GA et al. Sickle cell retinopathy and hemoglobinopathies. In: Joussem AM et al. Retinal Vascular Diseases. Berlin: Springer-Verlag; 2007. doi:10.1007/978-3-540-29542-6.
8 Yawn BP et al. JAMA. 2014;312(10):1033-1048.
9 Han IC et al. Retina. Published online Jan. 30, 2018. doi:10.1097/IAE.0000000000002057.
10 Lim JI, Cao D. Am J Ophthalmol. 2018;192:229-238.
11 Hoang QV et al. Am J Ophthalmol. 2011;151:990-994.
12 Jin J et al. J AAPOS. 2018;22(3):197-201.
13 Han IC et al. Ophthalmology Retina. 2018;2(6):599-605.
14 Myint KT et al. Cochrane Database Syst Rev. 2015 Oct 9;(10):CD010790.
15 Cai C et al. J Vitreoretin Dis. 2018;2(1):32-38.
___________________________
Dr. Akhter is a resident at the Kresge Eye Institute, Wayne State University, in Detroit. Dr. Latting is a clinical fellow at Bascom Palmer Eye Institute, in Miami. Dr. Scott is an associate professor of ophthalmology at the Wilmer Eye Institute, Johns Hopkins University School of Medicine, in Baltimore. Relevant financial disclosures: None.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Akhter None.
Dr. Latting None.
Dr. Scott Allergan: C.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|