Download PDF
A small phase 1 clinical trial has added greater confidence in the quest to treat 2 rare inherited retinal degenerative diseases caused by RPE65 mutations: Leber congenital amaurosis (LCA) and severe early-childhood–onset retinal degeneration.1 Not only was the gene therapy found generally safe but it also yielded improvements in visual acuity (VA) or other visual functions in 9 of 12 patients.
In the trial, 8 adults and 4 children—aged 6 to 39 years—underwent vitrectomy and received a subretinal injection of an adeno-associated virus vector expressing normal RPE65 into retinal cells of the poorer-seeing eye at 1 of 2 doses. Patients were followed for 2 years, and the effectiveness of the treatment was evaluated by means of best-corrected VA (BCVA), visual field (VF), electroretinography, and quality-of-life questionnaires.
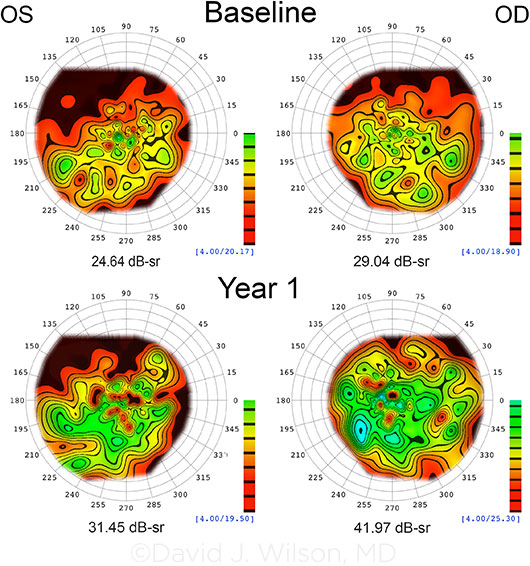 |
VISUAL FIELD MODELING AND ANALYSIS. Model represents VF changes seen in 1 trial subject from baseline to year 1. The right eye (OD) received gene therapy. Topographic iso-sensitivity lines have been drawn at 2-dB intervals. The calibration scale depicts the color coding for the iso-sensitivity lines, and the number just below the scale is the maximum sensitivity for the eye. The total volume in decibel-steradians (dB-sr) of the hill of vision (Vtot) is indicated below each model. Vtot increased from 29.04 to 41.97 dB-sr in the OD and was accompanied by a marked decrease in nystagmus and improvement in fixation instability. Vtot increase in the OS is thought to be associated with the improvements in nystagmus and fixation.
|
Safety and effectiveness. Patients experienced no serious adverse events related to treatment. Common adverse events associated with the surgical procedure included subconjunctival hemorrhage in 8 patients and ocular hyperemia in 5 patients.
“The trial included several children, who had better baseline visual acuity and experienced better visual results than the adults,” said David J. Wilson, MD, study coauthor and director of the Oregon Health & Science University School of Medicine in Portland. This was significant, he said, given that most safety trials generally look at patients with advanced disease.
At the 2-year visit, the 4 pediatric patients, whose baseline BCVAs were between 40 and 62 ETDRS letters, showed 6- to 14-letter increases in the treated eye. However, only 1 adult, who had a baseline BCVA between 20 and 31 ETDRS letters, experienced a 2.5-letter increase, while adults with baseline VA limited to counting fingers or hand movements showed no change in BCVA.
Apart from the VA improvements in 5 patients, other types of visual function improvements were reported in 9 of the 12 patients. These included increase in VF, loss of central scotoma, and subjective gain in vision in low light conditions.
Novel analytic tool. A unique feature of the trial was use of a novel VF analytic tool, called Visual Field Modeling and Analysis, developed by senior author Richard A. Weleber, MD. Compatible with a variety of VF devices, it allows accurate evaluation of both central and side vision, said Dr. Wilson. “Most visual field testing assesses the central visual field. But with many of these diseases, what is changing—and worth preserving—is the peripheral visual field. Having a tool that allows us to analyze that as an endpoint is very valuable.”
Implications for the future. Preclinical data suggest that retinal gene therapy has long-term effects, said Dr. Wilson, and the good responses seen in the children in this trial bode well for preventing progression of photoreceptor degeneration. Study participants will be followed for at least 15 years to more fully reveal the effects of therapy.
By proving safety, this trial helps advance the field, he said, and may speed FDA approval processes and make it easier to develop similar viral vectors for other single-gene diseases.
Over the next 10 to 15 years, there will likely be substantial changes in gene therapy, said Dr. Wilson, including refinements in both delivery techniques and vector technology. “Most important, researchers developing these therapies need to stay very objective,” he said. “Patients with these diseases are highly motivated, so we can’t rely on their subjective feedback about what the treatment accomplishes.”
—Annie Stuart
___________________________
1 Weleber RG et al. Ophthalmology. 2016;123(7):1606-1620.
___________________________
Relevant financial disclosures—Dr. Wilson: None.
For full disclosures and disclosure key, see below.
More from this month’s News in Review