By Rebecca Taylor, Contributing Writer, interviewing Kapil Bharti, PhD, Emily Y. Chew, MD, Baruch D. Kuppermann, MD, PhD, and David S. Liao, MD, PhD
Download PDF
Dry age-related macular degeneration (AMD) is traditionally thought to progress to two forms: geographic atrophy (GA) and neovascular AMD. But researchers are uncovering more nuanced approaches to dry AMD and laying the groundwork for novel treatment strategies.
Both types of dry AMD—with and without GA—are being studied, said Baruch D. Kuppermann, MD, PhD, at the University of California, Irvine. “While there is a continuum of disease, for clinical trial purposes it may be useful to consider them as separate patient pools with distinct types of treatment.”
And clinicians are keenly awaiting the outcomes of this research. “We don’t have a lot to offer patients with GA,” said David S. Liao, MD, PhD, in practice in Los Angeles. If treatments currently in development “prove safe and effective, then we’re going to have a fantastic opportunity to help a large number of people.”
Several approaches are showing promise, including hacking the complement cascade, repurposing a glaucoma drug, and coaxing induced pluripotent stem cells to become retinal pigment epithelium (RPE) cells.
 |
|
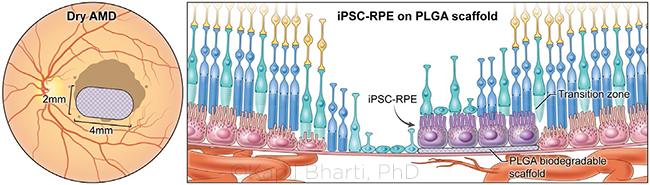
RPE PATCH. The 2 mm × 4 mm RPE patch is created from induced pluripotent stem cells (iPSCs), which are developed into RPE cells on a biodegradable polymer scaffold before being surgically transplanted behind the retina.
|
Blocking the Complement Cascade
The complement system augments the innate immune system through a central cascade of multiple factors, notably complement factor 3 (C3), the downstream complement factor 5 (C5), and further downstream factors C5a and C5b.
Pegcetacoplan. “Complement has been clearly identified in drusen, and there’s a lot of excitement around the science of complement inhibition in retinal disease,” said Dr. Kuppermann.
Pegcetacoplan (APL-2; Apellis) inhibits C3. In a phase 2 study, 246 patients were randomized 2:2:1:1 to receive intravitreal injections of the drug either monthly or every other month (EOM)—or sham injections on a monthly or EOM basis. At 12 months, GA growth rate was reduced by 20% with EOM injections and a more robust 29% with monthly injections of the active drug.1 APL-2 is now in global phase 3 trials with 1,200 patients.2
“Results showed a significant decrease in the growth of GA lesions with a clear dose/response effect,” said Dr. Liao. “Inhibition of the C3 molecule leads to a blockade of all complement pathways, slowing the progression of GA with ongoing treatment.”
A key concern is the process of conversion from dry to wet AMD. “An increased incidence of [new-onset] exudative AMD was seen in this study,” Dr. Liao said. Exudative AMD was identified more frequently in those who received monthly pegcetacoplan and in those who had a history of choroidal neovascularization (CNV) in the fellow eye.1 For instance, new-onset investigator-determined exudative AMD was detected in 20.9% (18/86) patients who received monthly pegcetacoplan, in 8.9% (7/79) who received the drug every other month, and in 1.2% (1/81) who received sham injections. Of note, patients who converted to wet AMD showed no change in best-corrected visual acuity at the time of diagnosis. In addition, Dr. Liao said, “visual outcomes with anti-VEGF treatment were good in those patients who did convert.”
Zimura. This drug “works in the complement pathway at C5a and C5b, where it directly inhibits formation of inflammasome and membrane attack complex (MAC), which cause cell death,” said Dr. Kuppermann.
In a phase 2/3 trial known as GATHER1, Zimura (avacincaptad pegol; Iveric bio) was administered to 286 patients.3 In part 1 of this study, patients were randomized in a 1:1:1 ratio to 1 mg Zimura (n = 26), 2 mg Zimura (n = 25), and sham (n = 26). In part 2, they were randomized in a 1:2:2 ratio to 2 mg Zimura (n = 42), 4 mg Zimura (n = 83), and sham (n = 84).
Both the 2- and 4-mg doses of the active drug met their primary efficacy endpoints. At 12 months, those who received 2- and 4-mg doses of Zimura, respectively, experienced 27% and 28% less growth in GA than did those who received sham treatments.3 At 18 months, that differential had increased to 28% and 30%, respectively, for the 2- and 4-mg doses of Zimura (vs. sham).4
No adverse events or cases of inflammation were reported through 18 months. However, choroidal neovascularization was observed in the study eyes of two patients who received 1 mg of Zimura (7.7%), eight who received 2 mg (11.9%), and 13 who received 4 mg (15.7%), as well as in three patients (2.7%) in the sham group.4
A confirmatory clinical trial, GATHER2, is currently enrolling patients; participants in this trial will be randomized to receive either Zimura 2 mg or sham.
Repurposing a Glaucoma Drug
In another approach, when brimonidine was delivered via a biodegradable intravitreal implant (Brimo DDS; Allergan) in a randomized phase 2 trial, it showed ability to reduce GA.5
“We injected the brimonidine implant every six months into eyes with geographic atrophy, and at 12 months it showed a robust effect, with a 29% decrease in the growth rate of lesions in the 132 μm-dose group [n = 49],” said Dr. Kuppermann. Those who received the higher-dose implant (264 μm, n = 41) had an even better result: reduction by 31%.5 A larger phase 2b study of 310 patients compared a 400-μm implant and sham injections every three months on patients with smaller GA lesions.6
The upshot? Larger lesions had the bigger benefit. “When the lesion size was 6 mm2 or greater, the growth rate was slowed down by 38%, which was found in a later analysis,” said Dr. Kuppermann. “The brimonidine implant seems safe, with a low rate of infection and/or inflammation, and it didn’t lower IOP at any of those doses.” A phase 3 study has been designed but is not yet initiated, he said.
“Brimo DDS seems to be both neuroprotective and cytoprotective,” Dr. Kuppermann said; thus, it appears to protect photoreceptors and retinal neurons as well as RPE cells.
Developing an RPE Patch
Central to GA pathogenesis is the RPE. “Between the GA lesion and the healthier part of the retina is the ‘transition zone,’ where the RPE cells are gone but the photoreceptors are still alive,” said Kapil Bharti, PhD, at the NEI. Past attempts to graft a patient’s older RPE tissue from a healthier part of the eye have had mixed results, he said. But “with the theory that RPE cell death precedes photoreceptor cell death, we wondered: If we transplanted RPE cells into that zone, would it stop photoreceptors from dying further?”
Enter the RPE patch. This novel approach creates autologous tissue from induced pluripotent stem cells (iPSCs). Like embryonic stem cells, these cells have the capacity to make any tissue. “They’re called ‘induced’ because you can make them from any adult tissue,” said Dr. Bharti. “This is the first time in the United States that anyone has isolated a few cells from a skin biopsy or blood and made patient tissue in a dish for clinical use, so you can imagine: The phase 1 FDA application was 12,500 pages long.”
Dr. Bharti’s lab converts iPSCs into RPE tissue that sits on a biodegradable scaffold and becomes the 2 mm × 4 mm RPE patch. “We start with 200 mL of a patient’s blood and reprogram the blood cells into iPSCs, which takes about three months to make including the quality control,” he said, “then we convert those iPSCs into RPE progenitor cells and put the progenitor cells on the scaffold.” As the scaffold degrades, the progenitor cells mature. “At the end of this process, they’re fully polarized, fully functioning RPE cells that have made their own membrane, by secreting the right proteins to replace the scaffold,” Dr. Bharti said.
The RPE is one cell-layer thick with a unique architecture. “On the basal side, the cells have a membrane separating the eye from the choroid blood supply, and the apical side has hair-like projections that ‘talk to’ the photoreceptors,” Dr. Bharti said. “We recreated all those apical and basal structures in a dish and did all kinds of assays to study how closely they resembled native RPE cells, and they had every physiological feature of native cells.”
A deep understanding of cell physiology and developmental cues is required to make these cells. “For the iPSCs to become RPE cells, a sequence of growth factors follows an intricate process, with the right growth factors going up and down in the right time, place, and concentration, to give rise to the given tissue,” Dr. Bharti said. “We can now recreate those processes in a dish using human stem cells.”
The implant procedure uses a three-port vitrectomy surgery developed by vitreoretinal surgeon Steve Charles, MD. “He helped us develop a tool that takes this tissue in the correct orientation, goes through the vitreous, and delivers it under the retina—and then, the retina is flattened on top of the transplant,” Dr. Bharti said.
Closer to Home: The Impact of Diet
While clinicians wait for dry AMD treatments, what concrete steps can be recommended to patients today?
“Diet plays a major role in macular degeneration, and it seems to be important in all stages” of the disease, said Emily Y. Chew, MD, at the NEI. Her review of data from the Age-Related Eye Disease Study 1 (AREDS1) and AREDS2 took advantage of the largest data pool available on macular degeneration with the longest follow-up ever conducted.1 “We had 13,204 eligible eyes in 7,756 participants with a 10-year follow-up, looking at diet and progression to late AMD, GA, and neovascular AMD,” Dr. Chew said.
The key takeaway? “Greater adherence to the Mediterranean diet—particularly fish intake—is associated with a lower risk of progression in eyes with different severity of AMD,” she said. “We found that if you have very early AMD, progression from the early to intermediate stage could be reduced by about 25% by eating a Mediterranean diet.” She added, “When we looked at patients in the intermediate group, a very high adherence to the Mediterranean diet had almost a 30% reduction in progression to late macular degeneration. It’s a dose/response effect: The more you follow this diet, the greater the benefit,” particularly with regard to GA.
Impact of genetics. Complement factor H may also play a synergistic role.2 “If you have complement factor H genetic changes and eat the Mediterranean diet, you get even more of a beneficial treatment effect,” Dr. Chew said.
If you make just one change. What one dietary change should ophthalmologists encourage their patients to adopt? “What really drove the results of the Mediterranean diet was eating fish,” she said. “Patients should consider eating fish twice a week.”
If you go full Mediterranean. The nine “eating points” from the Mediterranean diet are as follows: Decrease your intake of 1) red meat and 2) alcohol even as you increase your intake of 3) fish, 4) vegetables, 5) whole fruit, 6) whole grains, 7) nuts, 8) legumes, and 9) “good” fats. The latter, notably olive, walnut, and safflower oils, have a beneficial ratio of MUFA:SFA (monounsaturated fatty acid to saturated fatty acid).
And remember AREDS2 supplements. Dr. Chew’s work has also confirmed the benefits of the AREDS2 supplements.2 “They reduce the risk of developing vision-threatening late disease by about 25%,” Dr. Chew said. “We hope ophthalmologists are recommending this to their patients with intermediate AMD.”
__________________________
1 Keenan TD et al., for the AREDS1 and 2 Research Groups. Ophthalmology. 2020;127(11);1515-1520.
2 Chew EY. Am J Ophthalmol. 2020;217:335-347.
|
__________________________
1 Liao DS et al. Ophthalmology. 2020;127(2):186-196.
2 Puliafito CA, Wykoff CC. Int J Retin Vitr. 2020;6:18.
3 Jaffe GJ et al. Ophthalmology. Published online Sept. 1, 2020.
4 D’Amico DJ. Avacincaptad pegol, a novel C5 inhibitor, significantly reduces the mean rate of geographic atrophy growth in the phase 2/3 GATHER1 clinical trial. Presented at: AAO 2020 Virtual, Nov. 13, 2020.
5 Kuppermann BD et al. Retina. Published online March 3, 2020.
6 Freeman WR et al. Phase 2b study of brimonidine DDS: Potential novel treatment for geographic atrophy. Presented at: ARVO, April 28, 2019; Vancouver, British Columbia, Canada.
__________________________
Dr. Bharti is senior investigator, Ocular and Stem Cell Translational Research, at the NEI in Bethesda, Md. Financial disclosures: None.
Dr. Chew is director of the Division of Epidemiology and Clinical Applications and chief of the Clinical Trials Branch at the NEI in Bethesda, Md. She is also editor-in-chief of Ophthalmology Science. Financial disclosures: None.
Dr. Kuppermann is chair of ophthalmology, director of the Gavin Herbert Eye Institute, and codirector of the Center for Translational Vision Research at the University of California, Irvine. Financial disclosures: Alcon: C,S; Allegro: C,S; Allergan: C,S; Apellis: S; Aprea: C; Cell Care: C; Clearside: S; Dose: C; Eyedaptic: C; Galimedix: C; Genentech: C,S; GSK: S; Interface Biologics: C; Ionis: C; Iveric bio/Ophthotech: C,S; jCyte: C,S; Novartis: C,S; Oculis: C; Ocunexus: C; Regeneron: C,S; ReVana: C; Ripple Therapeutics: C; Theravance Biopharma: C.
Dr. Liao is a vitreoretinal specialist in private practice in Los Angeles. Financial disclosures: Apellis: S; Clearside: S; Genentech: S; Iveric bio: S; jCyte: S; Regeneron: S.
See the disclosure key at www.aao.org/eyenet/disclosures.