Download PDF
This month, News in Review highlights selected papers from the original papers sessions at AAO 2020 Virtual. Each was chosen by the session chairs because it presents important news or illustrates a trend in the field. For details of presentation times, check the Virtual Meeting Guide at either aao.org/2020 or in the virtual meeting platform.
Two years after a single gene therapy injection, patients with Leber hereditary optic neuropathy (LHON) have experienced significant visual improvement.1
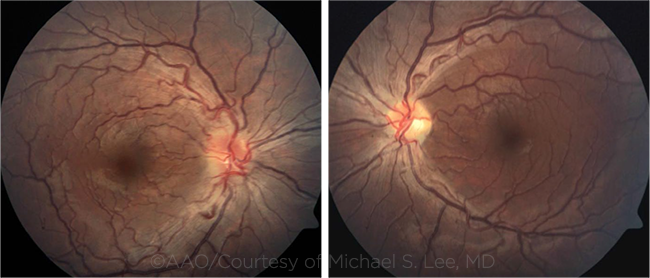 |
YOUNG PATIENT WITH LHON. Fundus photographs in this 17-year-old patient show hyperemic optic discs with blurred margins and moderately tortuous vasculature. The left optic disc shows mild temporal pallor.
|
Two clinical trials. In the two randomized sham-controlled phase 3 trials known as RESCUE and REVERSE, 76 patients with LHON received a single unilateral, intravitreal injection of an adeno-associated viral vector, rAAV2/ 2‑ND4 (Lumevoq, GenSight Biologics). They also received a simulated sham injection in their fellow eyes, said Patrick Yu-Wai-Man, MD, PhD, at the University of Cambridge, Moorfields Eye Hospital, and the University College London Institute of Ophthalmology.
The RESCUE trial evaluated patients who had onset of vision loss up to six months before treatment; the REVERSE trial evaluated those who experienced vision loss six to 12 months before treatment. The viral vector carries a replacement for the mitochondrial ND4 gene. When defective, this gene leads to rapid loss of retinal ganglion cells because of mitochondrial dysfunction; this is followed by degeneration of the optic nerves with visual failure.
VA outcomes. Visual acuity (VA) in the treated eyes improved by 26 EDTRS letters from the worst measured vision in the early-treatment group and by 28 letters in the late-treatment group, Dr. Yu-Wai-Man said. “The improvement that we observed is more than what would be expected based on the natural history of untreated patients,” he said.2
However, both the RESCUE and REVERSE trials produced an unexpected finding: The untreated contralateral eyes had VA gains comparable to those of the treated eyes.3 Two other LHON gene therapy research groups, in Miami and in Wuhan, China, have also reported this bilateral effect in their studies. Dr. Yu-Wai-Man will discuss possible reasons for this bilateral improvement in his AAO 2020 Virtual presentation.
European marketing. In September, GenSight Biologics submitted a marketing application for Lumevoq to the European Medicines Agency. To date, nearly 200 patients have been treated with the viral vector through trials and compassionate-use exceptions.
—Linda Roach
___________________________
1 Yu-Wai-Man P et al. Efficacy of gene therapy for Leber hereditary optic neuropathy: Final results of the phase 3 RESCUE and REVERSE trials. Presented at: AAO 2020 Virtual; Nov. 13-15, 2020.
2 Newman NJ et al. J Neuroophthalmol. In press.
3 Yu-Wai-Man P et al. Sci Transl Med. In press.
___________________________
Relevant financial disclosures—Dr. Yu-Wai-Man: GenSight Biologics: C.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Nakazawa Canon: S; Daiichi-Sankyo: S; Kowa: S; Nidek: S; Rhoto Pharmaceutical: S; Santen: C,L,S; Senju Pharmaceutical: C,L,S: Tomey: S; Topcon: S; Wakamoto Pharmaceutical: S.
Dr. Riemann AGTC: S; Alcon: C,L,S; Alimera: C,LS; Alimera Deutschland: C,L; Allergan: L,S; Aniridia Foundation International: C; Animal Eye Institute: C; Aerpio: S; Bausch + Lomb/Valeant: C,L; BioTime/Lineage: C,S; BMC/Eyetube: C; Chengdu Kanghong: S; CSTLII: L; Chruman Research: O; Clearside: S; Clovernook Center for the Blind and Visually Impaired: C; CVP (CEI Vision Partners): O; Digital Surgery Systems: O; Gore: C; Genentech/Roche: S; Gyroscope: C, S; Haag-Streit: C; Haag-Streit Surgical: C; Haag-Streit USA: C,L,P; HumanOptics: C; IamC2: C,P; iVeena: C,O; Janssen /Johnson & Johnson: C,P,S; Kaleidoscope Engineering: C,P; Lowy-MacTel Registry: S; Med One: C,P; Macor Industries: O; Northmark Pharmacy: O; Neurotech: S; Nightstar/Biogen: S; Novartis: S; Notal Vision: C,S; Novartis: L; Opthotech/Iveric: S; Orbit BioMedical: C; Regeneron: L,S; Reliance Industries: C,L,P; SalutarisMD: C,L; Spark: S; TrueVision: C,L,P; VEO: O; Vortex Surgical: C,O,P.
Dr. Shousha NEI: S; Research to Prevent Blindness: S; Resolve Ophthalmics: O. Related patents and PCT are owned by the University of Miami and licensed to Resolve Ophthalmics.
Dr. Yu-Wai-Man GenSight Biologics: C.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|
More from this month’s News in Review