Download PDF
The dream of using radiation to treat the wet form of age-related macular degeneration (AMD) is back. And, just as before, beginning with studies in the 1990s, a blast of early enthusiasm is being tempered by the painstaking process of attempting to prove clinical efficacy. This time around, though, no one is asking radiation to conquer choroidal neovascularization (CNV) solo.
Instead, researchers are asking whether ionizing radiation might work synergistically with intravitreal anti-VEGF drugs to reduce the personal, financial, and social burdens of treating AMD. They want to know if a primary or secondary radiation treatment might dry up CNV lesions faster, preserve visual acuity, and extend the intervals between intravitreal injections.
Early results of small uncontrolled studies seemed promising, but reports from two large randomized controlled clinical trials in the last year brought mixed results.
|
Radiation Zones
|
 |
|
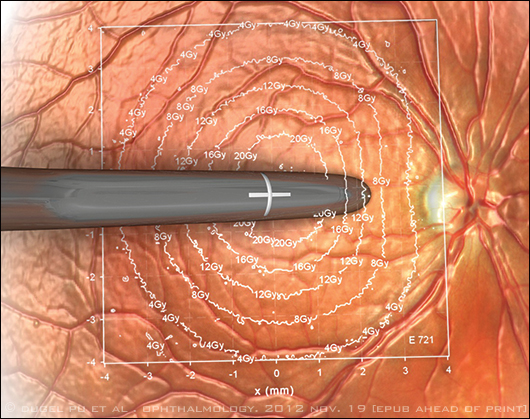
Schematic drawing depicts the amount of beta radiation delivered based on distance from the endoscopic probe used in epimacular brachytherapy. The point directly under the probe receives a dose of 24 Gy.
|
Recent Clinical Trial Results
CABERNET. The results were disappointing in the two-year follow-up report from CABERNET, a trial of epimacular brachytherapy. The study compared outcomes in treatment-naive patients who received localized beta radiation of 24 gray (Gy) plus anti-VEGF injections against outcomes in an injections-only control group. The study found that 77 percent of subjects in the radiation-treated group lost fewer than 15 EDTRS letters of visual acuity over the 24-month follow-up period, compared with 90 percent in the controls, the investigators reported last November.1
“After two years of follow-up, the safety profile appears acceptable. But we can’t recommend this as a primary form of treatment for CNV,” said lead author and coinvestigator Pravin U. Dugel, MD, managing partner at Retinal Consultants of Arizona, in Phoenix. “Regardless of the encouraging results in the smaller studies, you have to go by the science. The bottom line is that the CABERNET study did not meet its primary endpoint.”
INTREPID. At the Academy’s 2012 Joint Meeting in Chicago, Timothy L. Jackson, PhD, FRCOphth, reported positive results from the European INTREPID trial of an x-ray–based treatment, stereotactic radiotherapy.2 The study found that previously treated AMD patients whose maculae received 16- or 24-Gy radiation doses at the start of the study required 30 to 35 percent fewer as-needed (PRN) injections of ranibizumab in the 12 subsequent months than did the sham-treated controls (p ≤ 0.013), he said.
“The trial pretty convincingly shows that there is significant reduction in the number of injections, and it is suggestive that the visual acuity might possibly be a bit better, but certainly it doesn’t appear to be any worse,” said Dr. Jackson, who is a consultant vitreoretinal surgeon and HEFCE senior clinical lecturer at King’s College Hospital, London.
(click to expand)
Three Approaches to Treatment
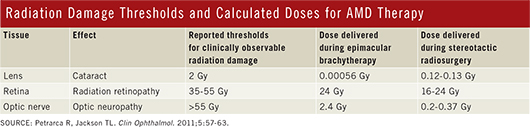
Currently, three systems for irradiating CNV lesions are in some stage of clinical testing. System designers had to find a balance between two competing objectives: dosing the aberrant blood vessels of AMD with enough ionizing radiation to cause them to regress, and avoiding radiation damage to the adjacent retina and other ocular structures. Each device maker balanced these objectives a different way—and made trade-offs along the way. None of the following devices has yet received FDA approval, but the Vidion and IRay systems have earned the CE mark.
Vidion Anti-Neovascular Epimacular Brachytherapy (EMBT) System. The Vidion system, formerly known as EpiRad 90 (NeoVista), is the most invasive of the three systems, as it begins with a pars plana vitrectomy. The vitrectomy enables the surgeon to visualize the affected area and to place the endoscopic probe’s tip on the internal limiting membrane directly overlying the area of disease activity. The 24-Gy dose of beta radiation is delivered in about four minutes.
The choice of a beta-emitting isotope, strontium 90/yttrium 90 (90Sr/90Y), minimizes the exposure of healthy ocular tissue to stray radiation because beta energy levels diminish quickly with distance (Fig. 1). “The dose is highest at the center, and then it falls off in a roughly exponential manner such that the dose received by the optic nerve and the lens is low,” Dr. Jackson said.
But this characteristic of beta radiation also means that small deviations in tip placement introduce dosing variability and possibly affect the procedure’s effectiveness, Dr. Dugel noted. He speculated that this might help explain why some patients in the CABERNET trial did poorly, while others did not require any intravitreal injections at all for two years.
“Even 0.1 mm away, there’s a 10 percent decrease in the energy. This is a great advantage if you want safety. But if you don’t place the probe where it’s supposed to be placed, you don’t get the advantage of radiation,” Dr. Dugel said. “It requires the angled tip of the probe to touch the retina. Some surgeons were placing the probe properly, and, by and large, their results tended to be much better.”
IRay Radiotherapy System. The IRay system (Oraya Therapeutics) delivers stereotactic x-ray radiation to the fovea through a computer-controlled device. The treatment is intended to be performed in the physician’s office and takes about 20 minutes.
After the eye is coupled to the system with a stabilizing mechanism, a robotically controlled x-ray source sends three overlapping, low-voltage beams, 4 mm in diameter, through the inferior pars plana to the macula. This route avoids the lens. Based on trial results, the total delivered dose is 16 Gy, down from the previous 24 Gy.
Unlike the other devices discussed in this article, radiation is transmitted from outside the eye. Thus, x-ray energy passes through other ocular structures before reaching the macula and could potentially cause them late radiation damage. Thus far, no such damage has been reported in the recent clinical trials.
“INTREPID has shown no radiation retinopathy out to 12 months, but it is very important to understand that radiation retinopathy more commonly occurs two or three years after treatment, so further safety follow-up is essential,” Dr. Jackson said.
Late last year, researchers reported promising 12-month results of “radiation-first” therapy with the IRay plus PRN ranibizumab. Although designed mainly as a phase 1 safety trial in 13 newly diagnosed subjects, the trial found that a single 16 Gy x-ray dose stabilized the CNV and kept loss of visual acuity to less than 15 letters in 11 subjects. The 13 patients received a total of 31 intravitreal injections, out of a possible 156 they might have had with injection-only treatment in one year.3 There were no serious radiation-related adverse events.
Episcleral brachytherapy. In January, Salutaris Medical Devices, of Tucson, Ariz., announced that researchers at Moorfields Eye Hospital in London have agreed to oversee a multicenter phase 1/2 clinical trial this year of SMD-1, a minimally invasive device for treating CNV with beta irradiation.
Episcleral treatment is more invasive than stereotactic radiotherapy because it requires a small conjunctival incision, but less invasive than EMBT because it does not involve a vitrectomy, said Reid F. Schindler, MD, in practice at Retina Specialists of Southern Arizona, and a clinical associate professor of ophthalmology at the University of Arizona, in Tucson. Dr. Schindler advised the company on the system design and is involved with its clinical trial planning.
To treat CNV, the physician threads a cannula between the sclera and Tenon’s capsule, guided by indirect ophthalmoscopic visualization of transilluminated light from a fiberoptic in the tip. The light enables the radioisotope inside the cannula to be placed directly underneath the CNV lesion to deliver a precisely aimed dose of beta radiation (90Sr/90Y), Dr. Schindler said. The total procedure time is about 15 minutes.
The only clinical trial of the device so far was a 90-day safety study of radiation plus two anti-VEGF injections, with a third allowed if needed. At 90 days, four of the six previously treated subjects had gained at least 15 ETDRS letters of acuity, he said.
Dr. Schindler continued to follow the six subjects after the study ended. “Out of the six people, there have been two that are just doing really well more than a year after their treatment, two who didn’t respond to the therapy, and two in the middle,” he said. “But those first two have not had any injections in more than a year, not since the two initial induction doses we required for the study.”
Dr. Schindler, whose past experience includes being an investigator in the Collaborative Ocular Melanoma Study, said that despite the deficiencies inherent in such a small and limited study, the results were encouraging. “Especially since it’s a relatively simple process,” he said. “It’s not very invasive, it possibly could be done in the office, and it potentially delivers a very accurate dose of radiation to the site we’re treating, with minimal nontarget tissue exposure.”
Barriers to Clinical Adoption
The case has not yet been made for radiation in the treatment of AMD, “given that treatment with Avastin or Lucentis is effective in 90 to 96 percent of patients,” according to Donald S. Fong, MD, MPH. Of particular concern is that some of these modalities are “relatively aggressive,” especially EMBT, as it requires vitrectomy, which increases risk of complications, said Dr. Fong, who is a retina specialist and epidemiologist at Kaiser Permanente Southern California, in Baldwin Park.
In addition, the socioeconomic impact of any new therapy should be considered,he said: “When an Avastin injection costs $20—and at a time when we are trying to expand health care coverage and reduce expenses—it’s very hard to see the rationale for taking on the substantial costs of a new technology that provides only a small incremental gain in reducing the number of injections.” He added that studies have already demonstrated an effective way to decrease the frequency of injections: the treat-and-extend regimens.
The question of greatest interest to Dr. Fong is whether radiation therapy can help the small percentage of patients who fail to respond to anti-VEGF treatment. Thus far, he said, the evidence is lacking; he hopes to see larger trials that can better delineate the potential usefulness of radiation in the retina clinic.
|
Next: Finding Out More Details
This year, NeoVista’s EMBT system will get a second chance with the anticipated release of 12-month results from the MERLOT trial, sponsored by the British National Health Service. “Given the results of CABERNET, all eyes are now on the MERLOT study. MERLOT is a large randomized controlled trial of EMBT for chronic active wet AMD,” said Dr. Jackson, who is the chief investigator. “Unlike CABERNET, which had a more intensive anti-VEGF dosing regimen in the control arm, both the radiotherapy and control arms in MERLOT have identical anti-VEGF regimens, so we will be better able to isolate the effect of radiotherapy and determine if EMBT is appropriate as a second-line treatment,” he said. The study includes 363 subjects who had persistent CNV despite anti-VEGF injections. “MERLOT is going to be critical in defining how this device is used,” he said.
Dr. Dugel agreed. “As of now, epimacular brachytherapy has missed its primary endpoint for treatment-naive patients in the CABERNET study. We need to wait for the MERLOT study to see if there is a role for this device in previously treated patients.”
Even if MERLOT brings more disappointing results, however, investigations of other delivery methods will keep radiotherapy on the ophthalmic research agenda. Low-dose radiation simply makes sense as a way to stop the rapid proliferation of cells anywhere in the body, said Dr. Dugel. “If you look at the systemic literature, it supports the synergistic effect of radiation with anti-VEGF therapy. It makes good physiological sense.” Beyond that, he added, there is a real unmet need, based on our current unsustainable treatment burden.
___________________________
1 Dugel PU et al. Ophthalmology. 2012 Nov 19. [Epub ahead of print]
2 Jackson TL. Responder Analysis of the INTREPID Study of Stereotactic Radiotherapy for Wet Age-Related Macular Degeneration Presented at: Retina Subspecialty Day; Nov. 9-10, 2012; Chicago.
3 Moshfeghi A et al. Br J Ophthalmol. 2012;96(10):1320-1324.
___________________________
Dr. Dugel is a consultant for Abbott Medical Optics, Alcon, Allergan, ArcticDx, Genentech, Macusight, Neovista, ORA, ThromboGenics, and Regeneron. He holds equity interest in ArcticDx, Macusight, and NeoVista. Dr. Fong is a consultant for ThromboGenics and receives grant support from Allergan. Dr. Jackson is a consultant to Alimera, Bausch + Lomb, and ThromboGenics. He has received lecture fees from ThromboGenics, support to present at meetings from DORC International and Ora-ya, and grant support from Allergan, NeoVista, Novartis, Oraya, and Retinal Implant. Dr. Schindler is an equity owner in Salutaris Medical Devices.