Download PDF
The intraocular inflammation that may occur after intravitreal therapy with brolucizumab (Beovu) can also be accompanied by retinal vasculitis severe enough to cause profound loss of vision, researchers have found.1
Real-world outcomes. This retrospective analysis of retinal vasculitis in 15 eyes of 12 patients from 10 U.S. centers was the first case series published in a peer-reviewed journal since isolated reports of brolucizumab-associated problems began emerging earlier this year.2-4
The patients’ mean visual acuity (VA) before treatment with brolucizumab was 20/53. By the time retinal vasculitis was diagnosed, it was 20/191 (range, 20/25 to 20/1,600). And at a mean of 25 days following diagnosis and treatment, it was 20/136. Nine eyes (60%) lost 3 lines or more, and five eyes (33%) had VA of less than 20/200.
The vasculitis and intraocular inflammation noted in these eyes ranged from “peripheral vasculitis to occlusion of large retinal arteries around the optic nerve or macula with severe vision loss,” the researchers said. All 12 affected patients were women, which suggests that autoimmunity may be a factor, said coauthor Scott D. Walter MD, MSc, at Retina Consultants in Hartford, Connecticut.
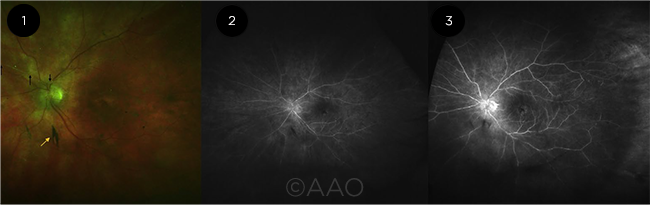 |
PROGRESSION. After bilateral brolucizumab injections, this patient experienced vitritis progressing to vasculitis despite treatment with oral and topical steroids. (1) Vitreous opacity (yellow arrow), optic nerve edema, and superior retinal artery sheathing (black arrow) are evident. (2) Globally sclerotic retinal arteries with peripheral nonperfusion are seen on early fluorescein angiography (FA). (3) Late-phase FA demonstrates hyperfluorescence from the optic nerve and perifoveal region, diffuse vascular staining, and peripheral vascular nonperfusion.
|
Insidious onset. These adverse outcomes occurred in a pattern distinct from anything ever seen with other approved anti-VEGF drugs, said Dr. Walter, also at the University of Connecticut School of Medicine in Farmington. Specifically, the inflammation associated with brolucizumab “tends to be milder in its early stages and more insidious in onset,” he said. “The patient might not become symptomatic for several weeks after the injection, and the inflammation may be mild enough that patient wouldn’t think to call the office.”
In some patients, “the inflammation was picked up when they returned for a scheduled injection,” Dr. Walter noted. In others, he said, “It was overlooked because there was no intense vitritis or hypopyon.” These are typical signs of intraocular inflammation associated with other anti-VEGF drugs, with onset typically in the first week after injection, he said.
Additionally, “There were multiple exposures to the drug in some cases, and a delay of weeks, as opposed to days, before the onset of clinically apparent intraocular inflammation and retinal vasculitis,” Dr. Walter said. “And if you miss catching this, then it can really get you into trouble.”
If you use brolucizumab. Retina specialists should be alert for inflammation and other events when using brolucizumab, the study authors said. And while researchers try to discover the mechanism behind the problems, Dr. Walter said that he has decided against starting his patients with age-related macular degeneration on brolucizumab, and that he is encouraging those already on it to switch to another anti-VEGF agent.
But for those clinicians who do use the drug, Dr. Walter advises a complete examination of both the anterior and posterior segments to evaluate for subtle signs of inflammation—even for apparently asymptomatic patients—before each subsequent injection. “The most important thing for anyone treating these patients is to not reinject an eye that has active inflammation with brolucizumab or any other anti-VEGF drug.”
—Linda Roach
___________________________
1 Baumal CR et al. Ophthalmology. Published online April 25, 2020.
2 Haug SJ et al. Am J Ophthalmol Case Rep. 2020;18:100680.
3 Jain A et al. Am J Ophthalmol Case Rep. 2020;18:100687.
4 Roach L. EyeNet Magazine. 2020;24(6):30-32.
___________________________
Relevant financial disclosures—Dr. Walter: Genentech: C.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Brooks None.
Dr. Milea Note: This study was funded by the Singapore National Medical Research Council (CS-IRG Grant) and the SingHealth Duke–NUS Ophthalmology and Visual Sciences Academic Clinical Program.
Dr. Swaroop None.
Dr. Walter Allergan: C; Castle Biosciences: C; Genentech: C.
Dr. Wong Allergan: C; Bayer: C; Boehringer-Ingelheim: C; EyRis: O; Genentech: C; Merck: C; Novartis: C; Oxurion: C; plano: O; Roche: C; Samsung: C. Note: This study was funded by the Singapore National Medical Research Council (CS-IRG Grant) and the SingHealth Duke–NUS Ophthalmology and Visual Sciences Academic Clinical Program.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|
More from this month’s News in Review