Download PDF
The internal limiting membrane (ILM) peel has been the gold standard to treat small, full-thickness macular holes since it was introduced in 1997.1 But what happens when primary surgery fails? Do the latest surgical techniques, building on the initial success of the ILM peel, have a role to play in repairing challenging macular holes?
Tackling Complex Cases
“With macular holes in general, we have a 95% to 96% success rate, but these are smaller holes, the patient’s first surgeries,” said Peter K. Kaiser, MD, at the Cleveland Clinic’s Cole Eye Institute. “The harder-to-fix holes are re-operations, very myopic or post-trauma holes, or longstanding holes.”
High myopia. When a macular hole occurs in patients with high myopia, these affected eyes “sometimes have a posterior staphyloma, which makes it harder for the retina to stretch and close the hole,” said John T. Thompson, MD, who practices in Baltimore. “Some have myopic macular schisis, a splitting of the retina around the hole,” which also makes it difficult to close.
Chronic or refractory. Chronic holes (those older than a year) and refractory holes (those with one or more failed surgeries) typically have lower success rates. For instance, with a chronic hole, you may have more traction, such as additional epiretinal membrane, said Tamer H. Mahmoud, MD, PhD, at the William Beaumont School of Medicine in Royal Oak, Michigan. “You may be able to close that hole by releasing the tractional forces, but functional improvement may be limited because of underlying retinal pigment epithelium [RPE] atrophy.”
Post-trauma. As for macular holes related to trauma, these can be very large with extensive RPE loss, Dr. Mahmoud said. In cases with a taut retina, tissue loss, and tangential traction, functional outcomes are reduced, and hole closure is limited, he added.
Other surgical challenges. Macular holes that present in patients with Alport syndrome or macular telangiectasia also can be problematic, as they limit the use of the ILM in repair, Dr. Mahmoud said. Another challenging scenario, said Chi-Chun Lai, MD, at the Chang Gung University College of Medicine in Taiwan, involves the macular hole with retinal detachment. And even accurate pre-op measurement of macular holes can be tricky (see “Shooting for Success”).
Novel solutions. Faced with these difficulties, retina surgeons have developed a series of novel surgical strategies. ILM flaps, autologous retinal transplants (ARTs), and amniotic membrane trans-plants (AMTs) have garnered the most attention. Other techniques, including lens capsule transplants and subretinal blebs, also are used (see “Four Additional Options”).
“The common thread for the most popular, successful techniques is to provide a scaffold of tissue beneath, within, or on the surface of the macular hole,” said Dr. Thompson.
 |
|
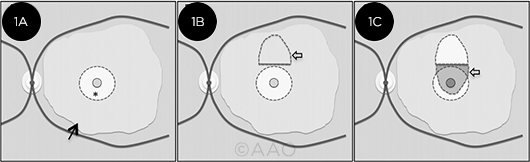
FLAP VARIATION. For the SWIFT technique, following ILM peel (1A), a strip of residual ILM forms the base of the flap (1B). The flap is then positioned over the hole (1C), covering the hole and the neurosensory retina. (* = central macular region; black arrow = residual stained ILM; lighter arrows = ILM flap and macular hole).
|
ILM Flaps
In 2010, Polish surgeon Zofia Michalewska reported that her inverted ILM flap technique improved both hole closure and vision outcomes for macular holes larger than 400 μm.1 Since then, a variety of ILM flap techniques employ the peeled ILM to serve as a scaffold to repair challenging macular holes.
Theme and variations. A number of ILM flap variations exist. Retina surgeons have more than 10 years of data on the ILM variants, said Dr. Lai. “They are very dependable and sustainable techniques to treat challenging macular holes.”
“The ILM flap technique is simple yet brilliant because you’re using tissue you were going to remove anyway, and it works very well for most complicated macular holes,” said Dr. Thompson. Results of histologic studies indicate that with a successfully closed macular hole, “a glial plug seals the hole and pulls its edges together so that the foveal photoreceptors are where they belong.”
With the flap techniques, the ILM becomes the tissue scaffold, whether it’s an inverted flap, a “retracting door” flap, or even what’s known as a “Texas taco” flap. (In the latter approach, the nasal ILM is peeled beyond the temporal edges of the hole, and the ILM flap is draped over the hole.1)
Inverted ILM flap. “For any of the atypical holes, we may seriously consider doing an ILM flap initially and not an ILM peel,” said Dr. Mahmoud. “We start by peeling the ILM, which disrupts the tangential traction, and use that ILM flap to cover the hole and help the retina bridge the gap.”
“You can do an ILM flap from any direction, but if you do it temporally, it’s by far the easiest. That’s because the vector forces, when you go to air, allow that flap to cover the hole without additional manipulation,” Dr. Kaiser said. “If you make it nasally, you have to use something to brush the flap over the hole.”
A global meta-analysis of the ILM peel and the inverted flap technique in 1,403 eyes with macular holes found the flap technique better at closing holes of all sizes, including those with retinal detachment.2 The inverted flap technique also resulted in greater BCVA improvement.
“Retracting door” ILM flap. For myopic patients, Dr. Mahmoud said, this technique is often his initial choice.3
“We start from the nasal side of the hole close to the optic disc, peel the ILM across the hole to the temporal side, then re-drape the ILM over the hole so it’s a hinged flap,” he said. “Because of the tangential traction from myopia, we’re removing all tractional forces around the hole, and the ILM retracts and covers the hole.”
He added, “We know from OCT angiography that the retina moves from temporal to nasal after gas tamponade, and since the flap moves from nasal to temporal where the base of the flap is, it closes the hole.”
SPOT technique. To boost the success of ILM flaps, Dr. Lai employs an approach that combines sub-perfluorocarbon liquid (PFCL) and an ophthalmic viscoelastic device (OVD). The OVD acts as a “glue” to hold the flap in place. This approach is known as SPOT (sub-PFCL OVD injection). In one study, the technique reduced the risk of foveal gliosis and resulted in 74% of flaps still being present at six months postoperatively.4
Retina surgeons use SPOT to ensure that the ILM flap isn’t dislodged during the procedure, said Dr. Lai. He also noted that he uses it to secure retinal transplants.
SWIFT technique. Another novel ILM flap technique is known as SWIFT (superior wide-base internal limiting membrane flap transposition). In a study of SWIFT in 17 eyes, it resulted in closure of myopic and chronic holes in 94% of eyes.5 Follow-up VA was at least 20/70 in 48%of eyes and 20/80 to 20/200 in 53% of eyes.
 |
|
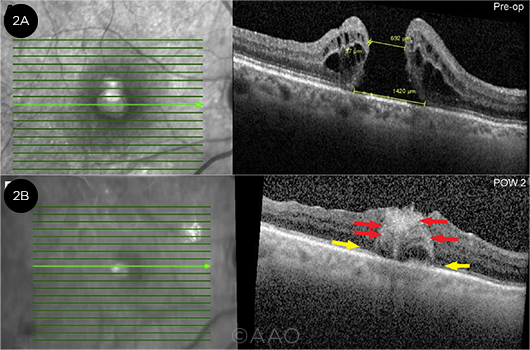
ART. (2A) Pre-op images show a macular hole with minimum diameter of 692 μm and maximum diameter of 1,420 μm. (2B) At post-op week 2 after ART, early integration of the transplant and partial reconstitution of the external limiting membrane and ellipsoid zone band (yellow arrows) are evident. (Red arrows = hyperreflective foci within the graft.)
|
Autologous Retinal Transplants
The ART procedure uses peripheral retina to close the macular hole.6
Using ART, “now we can close almost 100% of holes, and the beauty is not the procedure; it’s the biology, the plasticity of the retina,” said Dr. Mahmoud. On OCT, “initially, you see vertical lines between the graft and host, and the peripheral retina is thin. Over a few weeks, it starts thickening, and you see the alignment of the nuclear layer and plexiform layers. Eventually, you don’t even see the margins,” he said.
“Retina grows well with retina, so it integrates within the hole,” said Dr. Thompson. “Everything outside the macula is 20/200 vision or worse, so most patients don’t notice the blind spot created by the graft site.” He agreed that ART can close even difficult holes but cautioned that “we don’t know yet how well vision recovers.”
In a 2019 study of 41 eyes with refractory macular holes undergoing ART, 87.8% had complete anatomic closure. Mean corrected VA improved in 36.6% of eyes, stabilized in 41.5%, and worsened in 21.9%.7
In a global study in 2020, 33 surgeons pooled data from 130 macular holes repaired with ART. While case type varied—from large primary holes to refractory holes and holes with retinal detachments—outcomes appeared consistent. Overall, the closure rate was 89% (78.5% complete and 10% small eccentric defect), and 43% of patients gained 3 lines of vision, 29% gained 5 lines or more, and 12% achieved 20/50 vision or better.8
Cautionary notes. ART requires more skill and technical expertise than ILM flap techniques, said Dr. Lai. “Some publications say the graft should be 30% larger than the macular hole, which makes it difficult to measure.” In addition, he said, schisis of the graft has been observed.
Overall, Dr. Lai concluded, more long-term data are needed.
 |
|
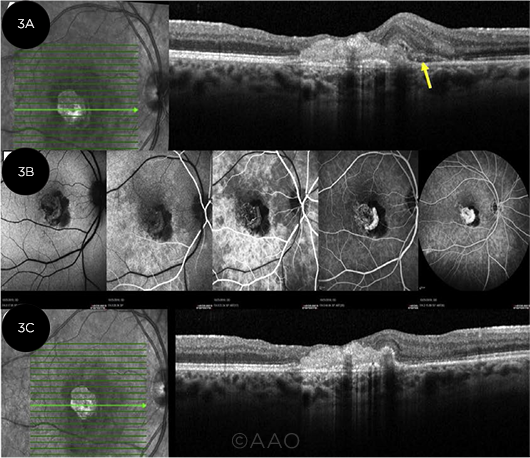
AMT. Late post-op images from AMT in a persistent macular hole. (3A) VA was counting fingers, and a retinal hemorrhage could be seen on exam (arrow). (3B) Images from fluorescein angiography show an area of blockage in the nasal fovea from the hemorrhage and leakage from a choroidal neovascular membrane. (3C) Following a series of anti-VEGF injections, VA improved to 20/150.
|
Amniotic Membrane Transplants
Human amniotic tissue provides more than mechanical scaffolding: It’s believed to spur the secretion of growth factors and RPE cell proliferation.1 In AMT, Dr. Thompson said, “you create a tiny punch of the amniotic membrane, fold it, and plug the hole.” He added, “Stan Rizzo in Italy deserves credit for this idea.”
In a 2020 study, 36 patients with failed macular hole surgeries underwent AMT. At three months, 35 holes were still closed, and all but three patients experienced improved BCVA by at least 1 Snellen line.9 Post-op imaging found gains in macular sensitivity and photoreceptors around the plug edges.
Cautionary notes. “The stem cells and trophic factors may hypothetically help with hole closure and be neuroprotective,” said Dr. Kaiser, “but AMT is not a magic cure-all.”
Moreover, as with any new technique, there may be unforeseen downsides to the procedure. “The amniotic membrane persists in the subretinal space,” said Dr. Mahmoud. Thus, over the long term, “it may prevent the diffusion of nutrients from the choroid and the RPE to the neurosensory retina.”
In addition, he noted, “Because of the mechanical adhesion and manipulation, you’re destroying the RPE and photoreceptors at the edges of the hole, and those cells are critical for functional recovery [seen on] microperimetry and multifocal electroretinography.”
 |
|
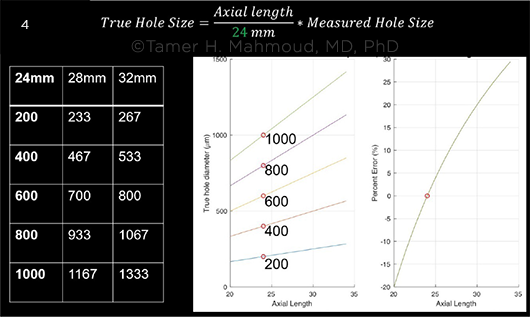
POTENTIAL ERROR. Transverse OCT measurements are subject to error in patients with long axial lengths. The graph at right shows the percentage of error for a hole that measured 400 μm on OCT with a 24-mm reference axial length. For the true measurement, multiply the OCT measurement by the true axial length and divide by 24, Dr. Mahmoud said.
|
Four Additional Options
Though the following techniques are less commonly used, they are potentially useful and worth considering, the experts said.
Lens capsule transplant. In this approach, the lens capsule serves as a scaffold, with a piece of the capsule used as a free flap. The creation of the capsular flap depends on the patient’s lens status: In pseudophakic patients, the surgeon removes the posterior capsule, Dr. Kaiser said. “For phakic patients, you can use the anterior capsule, which is considerably easier” from a technical standpoint.
In one study of 50 eyes with large macular holes, lens capsule transplants showed a 96% closure rate with vision improvement. However, 31 eyes in this study also received autologous platelet concentrate to reduce capsule dislocation.1
Autologous platelet concentrate (APC). “If a patient doesn’t have an ILM and you don’t have access to other options, you might try this,” said Dr. Kaiser. Autologous platelets contain growth factors and promote healing. However, Dr. Kaiser said, the use of APC is an older technique that is being replaced, “because if you use too much concentrate, it can ‘gel’ together and plug the hole, preventing closure.”
In a randomized study of injection of APC after vitrectomy for macular holes, those who received APC (n = 53) experienced a 98% closure rate compared to 82% for those who didn’t get injections of the concentrate (n = 57).1 APC has also shown anatomic and functional benefits when combined with ILM peels.1
Subretinal blebs. Subretinal injection of balanced salt solution (BSS) hypothetically releases adhesions between photoreceptors and the RPE.1
Oftentimes, chronic holes and those that have undergone multiple prior procedures are held open by scar tissue, Dr. Kaiser noted. The subretinal bleb technique “comes from doing translocation procedures in macular degeneration. It produces a localized retinal detachment around the hole, freeing up scar tissue and allowing the hole to close.”
Three small studies showed this technique achieved closure for persistent macular holes at rates from 85.4% to 90%.1 In contrast, a 24-month study comparing ILM flaps to subretinal blebs in the treatment of refractory holes showed 85.7% closure in the flaps versus 57.1% in the blebs, with better functional outcomes with the flaps.10
Retinal incisions. Another technique intended to resolve scar tissue involves cutting “relaxing” or “radial” incisions in the retina. In one small study, six eyes had incisions to repair large macular holes that had failed previous surgery. All told, five holes closed, and three patients saw vision improve.1
 |
|
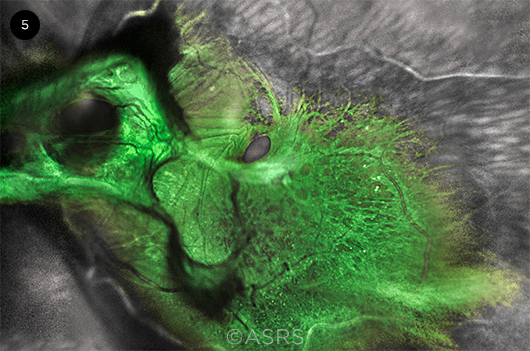
CONCURRENT. This 76-year-old patient presented with both a tractional retinal detachment and a macular hole. This image was originally published in the ASRS Retina Image Bank. Netan Choudhry, MD, FRCS(S), FASRS. Green Goblin Detachment. Retina Image Bank, 2022. Image Number 90269. © The American Society of Retina Specialists.
|
Shooting for Success
Pre-op planning. “For a patient with a hole smaller than 300 μm, we do an ILM peel,” said Dr. Mahmoud. “If it’s larger, we go for an ILM flap; and if it’s larger than 650 to 700 μm, my personal choice is an ART.”
“For the really big holes around 1,500 μm,” said Dr. Kaiser, “the flap techniques or the subretinal blebs won’t work, so you start to consider ART.”
“Often the hole enlarges if the first surgery fails, so these [chronic] holes are 600 to 1,000 μm,” said Dr. Thompson. “Because of the large gap in the macula, it’s harder to get the edges to come together.”
Measurement challenges. True measurement of the minimum linear diameter of macular holes poses its own challenges.11
“OCT is based on an eye-length of about 24 mm, but high myopes have longer eyes,” said Dr. Mahmoud. “You have to multiply the OCT measurement by the true axial length and divide by 24 to know the true measurement: For example, a 400-μm hole in a 30-mm eye is actually 500 μm. This underestimated size of myopic holes may partially explain the historically low closure rate with just an ILM peel.” Once adjusted to true size, he said, these holes can be matched to the best procedure for optimal outcomes.
Intraoperative decision-making. “Unlike a typical hole where the technique is standardized, in these situations you get into surgery and decide how to repair it, based on the anatomy you find while operating,” said Dr. Kaiser.
Dr. Thompson agreed. For instance, he said, the surgeon may have gone into surgery planning to do an ILM flap, but the flap breaks apart in an eye with a very thin ILM. “That’s when we switch to a transplant; these are game-time decisions based on what the eye is giving us.”
Post-op care. During follow-up, said Dr. Lai, it is important to watch for inflammation, infection, increase of IOP, a failed closure, or the patient’s inability to keep a prone position—although, as he noted, “most of the patients are very stable.”
With regard to prone positioning, Dr. Kaiser said, “I always get buy-in from patients: They need to be facedown for a week, for the majority of the day, period. This is the last dance; we need this surgery to work.”
Looking Ahead: Research Needs
To date, no trials have compared these surgical techniques head-to-head, said Dr. Kaiser. “Right now, each surgeon has a pet technique they publish on.” Moreover, he noted, “Currently, there are no imaging biomarkers or other features to help us predict which technique would work best with which hole.”
Issues under investigation. Investigators at Dr. Lai’s institution are developing a retinal “glue” to shorten the time patients need to spend in the prone position and doing basic research on the mechanism of wound healing, Dr. Lai said. “In the future we might introduce AI to predict [occurrence of] a macular hole, as patients are so worried about their fellow eye.”
Other researchers are investigating a clear, permanent vitreous substitute that could be used after removing the vitreous gel, said Dr. Mahmoud, as well as “working on stem cell and RPE transplants in the form of a sheet, which might be combined with the retinal transplant for better functional improvement.”
“A stem cell retinal patch would be a huge improvement in our field,” Dr. Thompson said. Also on his wish list: “A gas bubble that stays large for a prescribed time and then rapidly disappears, so you’d have a good tamponade until it’s no longer needed.” This would eliminate a second surgery to remove the silicone oil currently used as tamponade for some transplants, he said.
Bottom Line
Despite the work that remains to be done, radically improved outcomes for these surgeries mean that comprehensive ophthalmologists can now feel confident referring patients to repair older, challenging macular holes, said Dr. Kaiser. “In the past, the party line was that beyond two years, don’t bother sending them to a retina specialist—but now, you may be pleasantly surprised.”
___________________________
1 Cao JL, Kaiser PK. Ophthalmol Ther. 2021;10(1):1137-1153.
2 Marques RE et al. Ophthalmic Surg Lasers Imaging Retina. 2020;51(3):187-195.
3 Finn A, Mahmoud TH. Retina. 2019;39(Suppl 1):92-94.
4 Chou HD et al. Am J Ophthalmol. 2021;223:296-305.
5 Tabandeh H et al. Ophthalmol Retina. 2021;5(4):317-323.
6 Grewal DS, Mahmoud TH. JAMA Ophthalmol. 2016;134(2):229-230.
7 Grewal DS et al. Ophthalmology. 2019;126(10):1399-1408.
8 Moysidis SN et al. Ophthalmology. 2021;128(5):672-685.
9 Caporossi T et al. Sci Rep. 2020;10(1):18264.
10 Alezzandrini A et al. Int J Retina Vitreous. 2021;7(1):57.
11 Scoles D, Mahmoud TH. Ophthalmol Retina. 2022;6(2):95-96.
Meet the Experts
Peter K. Kaiser, MD Director of the Center for Ocular Research and Evaluation (CORE) at the Cole Eye Institute and professor of ophthalmology at the Cleveland Clinic Lerner College of Medicine, both in Cleveland. Relevant financial disclosures: Alcon: C; Bausch + Lomb: C; Carl Zeiss Meditec: C.
Chi-Chun Lai, MD Professor of ophthalmology at the Chang Gung University College of Medicine in Taiwan and superintendent at Chang Gung Memorial Hospital in Keelung, Taiwan. Relevant financial disclosures: Alcon: L; Allergan: L; Bayer: L,S; National Health Research Institutes of Taiwan: S; National Science Council of Taiwan: S; Novartis: L,S; Roche: S; Taiwan Liposome Company: S.
Tamer H. Mahmoud, MD, PhD Professor of ophthalmology at Oakland University William Beaumont School of Medicine and in practice with Associated Retinal Consultants, both in Royal Oak, Mich. Relevant financial disclosures: None.
John T. Thompson, MD In practice with Retina Specialists in Baltimore. He also is assistant professor of ophthalmology at the Wilmer Eye Institute and associate clinical professor at the University of Maryland in Baltimore. Relevant financial disclosures: EHR Command Center: O; Genentech: S; Ocutrx Vision Technologies: O.
Full Financial Disclosures
Dr. Kaiser Aerie: C; Aerpio: C; Allegro: C; Alcon: C; Bayer Healthcare Pharmaceuticals: C,L; Bausch + Lomb: C; Biogen: C; Boehringer: C; Carl Zeiss Meditec: C; Clearside: C; Eyevensys: C; Formycon: C; Galecto: C; Galimedix: C; Glaukos: C; iRenix: C; Iveric Bio: C,O; jCyte: C; Kala: C; Kanghong: C; Kodiak: C; Novartis: C,L; Omeros: C; Opthea: C; Oxurion: C; Regeneron: C,L; Regenxbio: C; Retinal Science: C,O; Santen: C; Stealth: C; Verana Health: O.
Dr. Lai Alcon: L; Allergan: L; Bayer: L,S; National Health Research Institutes of Taiwan: S; National Science Council of Taiwan: S; Novartis: L,S; Roche: S; Taiwan Liposome Company: S.
Dr. Mahmoud Aldeyra Therapeutics: S; NEI: S; Novartis: S; Regenxbio: S; Roche: S; Vortex: P.
Dr. Thompson EHR Command Center: O; Genentech: S; Ocutrx Vision Technologies: O.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|