Download PDF
Optical coherence tomography continues to dazzle researchers, with new software and hardware emerging at a blistering pace. An overview of where we are today.
David Huang, MD, PhD, and his colleagues were early participants in the optical coherence tomography (OCT) revolution. As a PhD student and member of a research team at the Massachusetts Institute of Technology, he was lead author of the first paper on the new technology. “We developed the first version of OCT at MIT in the 1990s and patented designs for time-domain and swept-source OCT,” said Dr. Huang, at the Casey Eye Institute in Portland, Ore. “The first prototype took several seconds to acquire an axial scan. Now commercial systems can acquire 100,000 axial scans per second, and laboratory prototypes can acquire millions of axial scans per second. It s amazing how far Moore s law of OCT has brought us.”
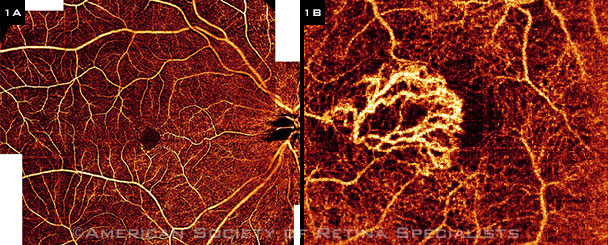
Each iteration of OCT technology continues to revolutionize research and clinical practice, allowing for increased understanding of pathogenesis, improved monitoring of progression, and prompt evaluation of treatment response. And the latest development, OCT angiography (OCT-A) is being touted by some as a fast, noninvasive, and 3-D alternative to 2 well-established tests—fluorescein angiography (FA) and indocyanine green (ICG) angiography (Figs. 1A, 1B). Here’s an overview of some recent developments in both hardware and software.
 |
|
(1A) This OCT-A image is of a 28-year-old woman without any macular pathology. Seven en face images were merged into a widefield view. (1B) This OCT-A image is of a 62-year-old man with wet AMD and type 2 neovascularization. A tree-like neovascular membrane is located above the RPE juxtafoveally.
|
Hardware: Choosing a Platform
Time-domain OCT (TD-OCT) is being surpassed by the newer platforms. Indeed, many experts now say that if you have time-domain technology, it’s time to upgrade.
For now, spectral-domain OCT and swept-source technology, which is only emerging in the United States, occupy much of the same real estate in the OCT world. Here’s a brief primer on some of the advantages and disadvantages of each (see also “Challenges of Imaging the Vitreous”).
Spectral-domain OCT. The broadband superluminescent diodes used in the manufacturing of SD-OCT instruments are commonly available and have a desirable price-to-performance ratio with a center wavelength near 820 nm, said Richard F. Spaide, MD, with Vitreous-Retina-Macula Consultants of New York City.
- Advantages. SD-OCT has eclipsed TD-OCT because it offers higher resolution, faster scan acquisition times, an increased area of retinal sampling, and 3-D imaging possibilities.
- Disadvantages. “The greatest limitation of SD-OCT is that the center 840 nm wavelength doesn’t do a good job of penetrating well through the retinal pigment epithelium [RPE] because of scattering by melanin granules,” said Philip J. Rosenfeld, MD, PhD, at the Bascom Palmer Eye Institute in Miami. SD-OCT’s other limitation is its maximal scanning speed of around 70,000 to 80,000 A-scans/second. (This refers to commercially available ophthalmic systems; laboratory systems can run much faster.)
Swept-source OCT. Although some people think of swept-source technology as the next generation of OCT, Dr. Huang said, it is actually an older idea already documented in the original MIT patent—it was just implemented later. SS-OCT offers a longer wavelength—1,050 nm—and has faster scanning speeds of 100,000 A-scans/second. (Again, this refers to commercial ophthalmic systems; faster speeds are being obtained with cardiovascular systems as well as ophthalmic systems under investigation.)
- Advantages. “The relative advantages of SS-OCT inherent in the system design are lower sensitivity roll-off with depth in the sample, a deeper imaging range, and no need for a grating with its associated light losses,” said Dr. Spaide.
- Disadvantages. SS-OCT’s disadvantages include “the need to compensate for nonlinear sweeps over the bandwidth of the light source and the potential generation of signals from mismatches in path length between the sample arm and the reference arm. This is known as coherence revival, which induces bright artifacts in the image that can be difficult to remove,” Dr. Spaide noted.
Which platform for which disease? Dr. Huang believes that SD-OCT is better suited for imaging retinal layers. But when looking into the choroid, SS-OCT might have some advantages. However, neither technology is necessarily better than the other, he said, and he predicted that both platforms will continue to be used. “SD-OCT tends to run on a shorter wavelength with better depth and transverse resolution. SS-OCT tends to run in the longer wavelength with better penetration through the RPE and choroid. Both have adequate imaging range for the retina.”
A note on wavelengths. Although SD-OCT and SS-OCT wavelengths are not separated by much numerically, certain types of scattering are sharply reduced even with minor changes in wavelength. “This allows greater penetration into tissue for longer wavelengths of light,” Dr. Spaide said. “However, reflection is intertwined with scatter; and, as a result, reduced scattering can cause reduced reflection [backscatter], which is what forms the image in OCT.”
Dr. Spaide added that although other wavelengths are available for swept-source lasers, they have limited utility because of the water absorbance of infrared light in the eye.
A note on scanning speed. Faster is better because it allows you to perform more repeats for each B-scan, said Dr. Rosenfeld. For instance, if an ophthalmologist wants to perform OCT-A, “That requires that multiple B-scan repeats be performed at 1 location, and then the B-scan position is moved, and 4 or 5 B-scan repeats are performed at the next location,” Dr. Rosenfeld said. “The difference in the intensity and/or phase information from each repeated scan at the same location is then calculated, and the difference in the signal is due to blood flow—because, in theory, this should be the only change between the repeated scans.”
Dr. Rosenfeld tells people that the scanning rate is like money: “You can spend your A-scans in different ways, depending on what you want. If you want more real estate, you can scan at a lower density over wider areas and get lower-resolution images with less capability of flow rate discrimination. Or you can scan a smaller parcel of real estate at a preferred location, get a higher-resolution image, and be able to detect a wider range of flow rates.”
A note on algorithms. Dr. Rosenfeld added that different algorithms calculate blood flow and may be referred to as intensity algorithms, phase algorithms, or complex algorithms (the latter incorporates both intensity and phase). “But the best algorithm to use is going to depend upon the instrument you have,” Dr. Rosenfeld said. “The more repeats that you can perform in a short period of time, the more sensitive you will be to detecting flow.”
Research Update: Macular Telangiectasia Type 2
The MacTel project, an international consortium, is working to understand the natural history of macular telangiectasia type 2 (MacTel2). And although ophthalmologists have used other forms of OCT to evaluate the condition, OCT-A may turn out to be an ideal technology for the job.1
The project is funded by the Lowy Medical Research Institute in La Jolla, Calif.; according to Dr. Rosenfeld, more than 1,000 patients are enrolled worldwide. The clinicians and researchers involved in the project are working to understand the natural history of the disease in hopes of developing a treatment.
Dr. Rosenfeld’s institution has more than 100 patients enrolled in the MacTel project, and because the disease affects only the central macula, it’s a good fit for OCT-A. In MacTel2, you can see the earliest manifestations structurally because the deep parafoveal retinal microvasculature is affected early, particularly on the temporal edge of the fovea.
“This disease is characterized by a dropout of the microvasculature,” Dr. Rosenfeld said. “On FA, leakage can be seen just temporal to the foveal center, and we and others have shown that OCT-A can demonstrate abnormalities in the capillaries. The microvasculature becomes dilated and develops aneurysmal changes that are similar to what is seen in diabetes.”
When the vessels start dropping out and disappearing, they become more dilated and tortuous, and the changes start to spread around the fovea, with abnormal anastomoses developing with the more superficial retinal capillaries. In the later stages, more microvascular loss and retinal atrophy occur, and the retinal anastomoses become more prominent, especially temporally. A late complication is the development of neovascularization arising from both the retinal and choroidal vasculature.
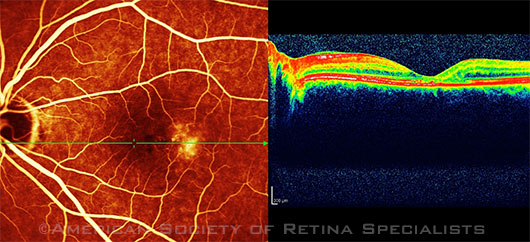
Scanning laser ophthalmoscopy and SD-OCT of a 56-year-old woman with MacTel2.
|
Dr. Rosenfeld added that OCT-A now allows the retina to be separated into different layers—the inner superficial retinal circulation and the deeper middle retinal circulation—and ophthalmologists have debated whether there are 2 or 4 layers of capillaries. What we do know, he said, is that “there are simply distinct layers of capillaries within the retina, and now they can be separated and examined individually.”
He added, “Ophthalmologists didn’t know the microvascular details about any of this until we could observe the changes in the absence of fluorescein leakage, which obscures many of the details when traditional FA is used. We speculated about these in vivo changes based on FA, but now, over the past 2 years since we’ve been using the research protocol, we have proof of what is happening.”
___________________________
1 Spaide RF et al. Ophthalmology. 2015;122(11):2261-2269.
|
Hardware + Software: Here Comes OCT-A
Dr. Spaide described OCT-A as a technology “that will transform the way we visualize retinal diseases.”
How it works. OCT-A may be based on either an SD-OCT or an SS-OCT platform. Early OCT-A research efforts required many repeat scans at the same location. New algorithms, like that in the AngioPlex OCT-A (Carl Zeiss Meditec), are faster. And the split-spectrum amplitude-decorrelation angiography (SSADA) algorithm developed by Dr. Huang and his colleagues requires only 1 repeat scan at each location and makes it possible to capture the entire macula in 3 seconds.1 Alternatively, SS-OCT devices, with their higher speed, also make it possible to scan the entire macula in approximately the same amount of time, said Nadia K. Waheed, MD, MPH, at Tufts Medical Center and the Boston Image Reading Center in Boston.
Evaluating blood flow. “We know that certain elements within the eye are completely static—like the photoreceptors, optic nerve, and macula—whereas other structures are constantly moving, including blood cells in the blood vessels,” said Dr. Waheed.
“All the various forms of OCT-A rely on rapid still shots from the back of the eye, and the computer algorithm analyzes what is different about these shots,” Dr. Waheed said. “If the patient is perfectly still, the only thing moving will be the blood. So when you subtract everything that’s still and everything that’s the same between the 2 images, what you have left is blood flow—and this gives us a way of looking at blood flow through the vessels without having to inject any dye.”
Scanning depth. OCT-A also allows ophthalmologists to scan depth. “It’s not just a 2-D image,” Dr. Waheed elaborated. “It’s a 3-D image, and this allows us to scan the surface blood vessels as well as the blood vessels underneath and to detect the flow pattern in 3 dimensions. With FA or ICG angiography, all you have is a 2-D shot. But because OCT itself is a 3-D imaging modality, it images the entire volume. This lets you compare one volume to another as opposed to one flat shot to the other, and you end up with a 3-D representation of the vascular flow inside the eye.”
Disease applications. Dr. Waheed said that her group at Tufts is applying OCT-A to the disease patterns they see in their patients, specifically age-related macular degeneration (AMD) and diabetic retinopathy. The researchers are also evaluating the reproducibility and repeatability of the various measures and evaluating which measures are of most importance in clinical trials. “The ultimate goal is to be able to use OCT-A as an early marker of response to therapeutic modalities that are being tested in clinical trials in a variety of settings, including exudative and non-exudative AMD and diabetes,” she said. (See also “Research Update: Macular Telangiectasia Type 2.”)
One advantage of OCT-A is that it is able to identify new blood vessels growing from the choroid in choroidal neovascularization and AMD, said Dr. Rosenfeld. “This perfectly corresponds with and coincides with what is seen with traditional angiographic imaging. We can see the neovascularization.” He added that he expects OCT-A to eventually replace FA and ICG angiography for neovascular lesions and diseases that affect the retinal and choroidal microvasculature.
Dr. Rosenfeld also contends that OCT-A will become the modality of choice for diseases of the optic nerve and central macula. “Right now, we have great visualization of the retinal and anterior choroidal vasculature, and because we get only small areas of high-resolution imaging, the best images cover a 3 × 3-mm area, which is ideal for optic nerve imaging and central macular imaging.”
“In an OCT angiogram, it is relatively easy to see choroidal neovascularization or retinal capillary dropout in diabetic retinopathy,” Dr. Huang added. “But in glaucoma, it’s more subtle—you have to develop software to quantify the vascular density and validate it in a large population database regarding what is normal and what is outside normal. As a result, it will take longer for OCT-A to become useful in glaucoma. But our preliminary results show promising indications that OCT-A will significantly advance glaucoma diagnosis and monitoring”.
A revolution in the clinic? Like many ophthalmologists, Dr. Rosenfeld initially was not convinced that OCT-A would have a significant impact. “The argument that I had been making was, ‘Show me the proof that OCT-A is going to change the way I manage patients.’ Even though I had been using a research OCT-A unit, it really hadn’t altered patient management compared with typical OCT,” he said.
“But we recently had a case in which I never would have ordered fluorescein or ICG angiography,” Dr. Rosenfeld said. “However, I saw dramatic neovascular progression in both of the patient’s eyes, and that changed my management. When I looked more closely at the volume of the retinal pigment epithelial detachments in both eyes, I saw a slight volumetric increase that we had shown was a harbinger of disease progression.”
He continued, “The change in the neovascular lesion was not subtle and should have been a much easier clinical indicator to follow when deciding when to re-treat. I was going to treat the patient and see her back in 8 weeks, but I changed my management and treated her every 4 weeks because she was clearly progressing. Her eyes responded by demonstrating a commensurate decrease in the volume of pigment epithelial detachment and in the size of the neovascular complex. I really didn’t appreciate the magnitude of disease progression when I just used conventional OCT.”
Dr. Rosenfeld added that OCT-A is not going to replace traditional modes of angiography for diseases outside the central macula. “But in the future, we’re just not going to use conventional angiography for diabetes, vein occlusions, AMD involving the central macula, or glaucoma.”
For his part, Dr. Spaide said, “I don’t see OCT-A as a replacement for FA or ICG angiography. OCT-A is making us aware of the limitations of dye-based angiography for many retinal diseases. Continuing along this path will allow us to learn about retinal diseases in ways that we couldn’t with dye-based angiography.” However, he said, “Dye-based angiography may still have uses even in a world with OCT-A.”
In summing up, Dr. Spaide noted, “We shouldn’t be constrained in our thinking to categorize OCT-A as a replacement for dye-based angiography. It is its own technology that has many opportunities.”
|
Challenges of Imaging the Vitreous
Adequately imaging the vitreous with OCT is turning out to be challenging, according to Dr. Spaide. “Because the vitreous is optically engineered to be transparent, the strength of the reflection is limited, and the ratio between the signal of the reflection and the noise inherent in imaging is low.”
Another challenge is that, in general, it’s not possible to average images of the vitreous unless the images are acquired over a very short period of time. “Because the vitreous moves all the time and changes in configuration, simple registration of the images is not possible,” Dr. Spaide noted. “However, it is possible to use a high-speed device and image line scans. It’s not possible to average volume scans yet because of motion and deformation of the vitreous.”
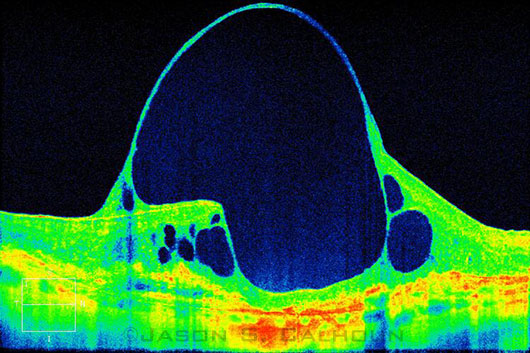
Enhanced-depth OCT image of a giant cyst in a patient with AMD.
|
Dr. Spaide offered the following additional observations:
Depth. Another factor affecting vitreous scanning is the limited scanning depth of OCT devices. Popular OCT instruments on the market have a scan depth as low as 1.9 mm. Because of the roll-off in sensitivity and the poor signal-to-noise ratio of vitreous imaging, SD-OCT is less likely to be useful for imaging the vitreous. In contrast, SS-OCT has the capability of imaging greater depths for more than one reason, the chief of which is the limited roll-off in sensitivity with depth. However, swept-source instruments have other challenges, including coherency revival, which can make bright artifacts in an image.
Image folding. Another aspect of deeper imaging is the possibility of image folding. This occurs when objects that are being imaged cross the zero-delay line. An upside-down version of the object is seen, and the image appears as if it is folded. This upside-down version is called the conjugate image and is inherent in the Fourier transform.
There are many ways to try to suppress or eliminate the conjugate image in order to expand the depth that the OCT instrument can scan, and doing so would double the range of an OCT instrument.
At present, all of these methods are capable of only incompletely suppressing the conjugate image. If the image produced a strong signal throughout, this might not be so objectionable. However, in vitreous imaging, the retina and choroid produce the conjugate image, while the vitreous doesn’t produce much of a signal. Therefore, it is difficult to avoid seeing an upside-down version of the retina floating in the vitreous with any method of conjugate suppression.
Focusing. The final limitation in imaging the vitreous is the way in which OCT instruments “focus.” The tissue is illuminated with light, and the reflections from tissue create the image. The light source enters the tissue as a funnel of light, comes down to a small disc, and expands from there. The maximal lateral resolution is obtained at the tightest focus of the light and nowhere else. As a result, maximal clarity and signal-to-noise ratio are obtained at one plane only. To overcome this problem, the focus of the light source can be swept through the tissue, and only the sharpest rendition of tissue can be recorded at one time. The resultant image contains the sharpest possible version of the imaging. This is called dynamic focusing and windowed averaging.
|
Software: En Face Imaging
Another recent development is the visualization approach known as en face OCT imaging, in which software is used to reconstruct C-scan images on the coronal plane. En face OCT images can be generated with any OCT technology, from TD-OCT to SS-OCT, and Dr. Waheed described it as the most “intuitive” way to visualize OCT-A.
Disease applications. En face OCT is being used to view both the anterior and posterior segments of the eye. For instance, in the anterior segment, researchers are assessing its facility in evaluating corneal dystrophies2 and comparing bleb characteristics after filtering surgery.3 And in the posterior segment, recent studies have described en face results in patients with cystoid macular edema (CME)4 and geographic atrophy,5 and researchers have used it to visualize pathologic features of the choroid.6
A revolution in the clinic? “Looking at en face images can provide a better appreciation of how serious a problem is—how much area it covers and how it correlates with what you see on the fundus [exam],” Dr. Huang said. “En face visualization of geographic atrophy or drusen is very useful in assessing the severity of dry AMD.”
En face imaging also is helpful when looking at CME or a macular hole, he said. It can help you understand how big the hole is or how widespread the cystoid edema is. “But you still need the cross-sectional image to determine whether it’s a partial-thickness hole or a full-thickness hole. These are situations in which you would need to use both approaches.”
Still a few kinks to work out. Researchers and clinicians who are interested in widefield OCT en face angiography are going to have to wait, Dr. Rosenfeld said. “Until we get faster scanning rates, better detection hardware, and more advances in software, we are not going to be able to do high-resolution widefield OCT en face angiography,” Dr. Rosenfeld said.
And Dr. Spaide cited some potential limitations of en face imaging: “The 2 more important ones are collapse of the depth information and the possibility of segmentation errors.” Volume rendering may be a solution, as it “does not lose the depth information and does not directly require segmentation,” he said.
Ready to Take the Plunge?
Each new research development brings a corresponding introduction of new hardware. The U.S. Food and Drug Administration cleared the Zeiss AngioPlex OCT-A system in September; similar approval of Optovue’s AngioVue OCT-A system is expected soon.
Dr. Spaide has soothing words for ophthalmologists who are wrestling with OCT purchase anxiety. “Sometimes people concentrate on 1 or 2 aspects of the differences in SS-OCT and SD-OCT, and they don’t look at what the 2 technologies have in common,” he said. “Other times, people make polarized statements or draw conclusions that aren’t constructive. To some people, a diesel automobile is much better than a gasoline-powered car. To others, the contrary is true. In reality, both modalities can make a fine car, and most people really couldn’t tell much of a difference.”
Right now the same is true for OCT instruments. “It’s possible to make a fine instrument” with either SD-OCT or SS-OCT, Dr. Spaide said. “There may be a winner in the horse race in the future, but right now—in a cost-for-performance limited environment—I wouldn’t sweat the details that much. Purchase of an OCT instrument should be based on how its capabilities match the needs at hand.”
___________________________
1 Jia Y et al. Opt Express. 2012;20(4):4710-4725.
2 Ghouali W et al. J Fr Ophtalmol. 2015;38(5):388-394.
3 Meziani L et al. J Glaucoma. Published online Sept. 14, 2015. doi: 10.1097/IJG.0000000000000319.
4 Murakami T et al. Invest Ophthalmol Vis Sci. 2012;53(3):1506-1511.
5 Nunes RP et al. Ophthalmic Surg Lasers Imaging Retina. 2013;44(4):344-359.
6 Ferrara D et al. Ophthalmology. 2014;121(3):719-726.
Meet the Experts
David Huang, MD, PhD Professor of ophthalmology at the Casey Eye Institute in Portland, Ore. Relevant financial disclosures: Carl Zeiss Meditec: P; Optovue: O,P,S.
Philip J. Rosenfeld, MD, PhD Professor of ophthalmology at Bascom Palmer Eye Institute in Miami. Relevant financial disclosures: Carl Zeiss Meditec: P,S.
Richard F. Spaide, MD In private practice at Vitreous-Retina-Macula Consultants of New York. Relevant financial disclosures: Genentech: C; Topcon: C,P.
Nadia K. Waheed, MD, MPH Assistant professor of ophthalmology at New England Eye Center and Tufts University School of Medicine in Boston and director of the Boston Image Reading Center. Relevant financial disclosures: Carl Zeiss Meditec: S; Thrombogenics: L.
For full disclosures and the disclosure key, see below.
|