This article is from March 2012 and may contain outdated material.
EDITOR’S NOTE: The March 2012 Retina CU on retinal prostheses incorrectly stated that clinical trials of Retina Implant are under way in the United States. A U.S. trial is indeed being planned, and Wills Eye Institute in Philadelphia, Penn., is signed on to be the lead site; but its start is contingent on FDA approval.
In early 2002, a team of surgeons headed by Mark S. Humayun MD, PhD, implanted an electronic device, which was called the Argus I, onto the surface of a blind man’s retina. The patient was then able to identify the direction of an object moving in front of the device’s spectacle-mounted camera. He didn’t see much—but he saw.
In an interview with USC Health Magazine,1 Dr. Humayun, professor of ophthalmology, biomedical engineering, and cell and neurobiology at the University of Southern California, said, “This is like the Wright Brothers. This is the first time we’ve been able to fly. It took a lot of work to get to this point, but this time, when we took off, we flew.”
This accomplishment represents an important milestone for a field that began in the 1980s and which now includes more than 15 companies and research groups in six countries. Those currently in or near human testing include Boston Retinal Implant Project, or BRIP (Boston), a cofounder of the field along with Second Sight (Sylmar, Calif.), Retina Implant AG (Reutlingen, Germany), Intelligent Medical Implant (Bonn, Germany) and Epi-Ret (Bonn).2 (Optobionics, which had an early chip, went bankrupt in 2007 and is now under reorganization.) Of these, the two groups that appear to be farthest along in development are Second Sight, which received Europe’s CE approval in 2011 to market the Argus II, and Retina Implant AG; prostheses from both companies are in human clinical trials in the United States.
Although these devices vary in terms of where the chip is placed within the eye, the number of electrodes on the chip, and operational mechanics, they all aim to translate light into electrical stimulation of the retina to generate artificial vision. Similar to the cochlear implant, they act as sensory replacements; in the case of retinal prostheses, they replace photoreceptors damaged by degenerative disorders such as retinitis pigmentosa or AMD.
Artificial vision is no longer a sci-fi fantasy. It’s here. And it’s commercially available in Europe. But is it ready for prime time? The answer depends on your assessment of the progress to date as well as the problems that lie ahead.
|
|
|
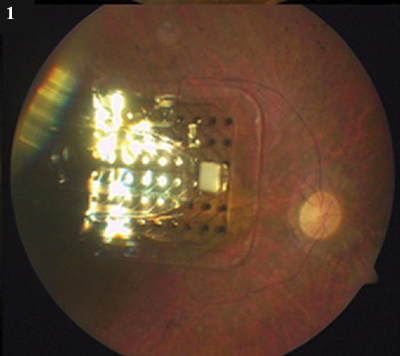
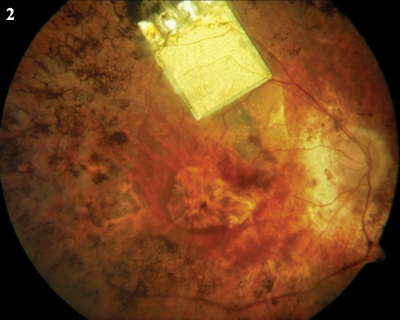
In the Eye. (1) The Argus II epiretinal chip. (2) The subretinal device from Retina Implant AG.
|
Progress: The Blind Can See
The two retinal prostheses that are furthest advanced in clinical trials have been shown to achieve some degree of vision, but is the quality of that vision sufficient to be truly useful to blind patients?
Second Sight’s Argus II. A flurry of reports at ARVO 2011 showed progress with Argus II. Dr. Humayun and colleagues presented an update from their clinical trial that is testing the device in 30 subjects with bare light perception or worse at 10 sites worldwide. According to their abstract, “Results on visual function tests with high-contrast stimuli showed a hierarchy of function, progressing from the ability to locate an object, through the ability to detect the direction of motion, and finally to the ability to distinguish the orientation of black and white gratings.”3 A presentation by other researchers showed that some subjects were also able to read four-word sentences with letters approximately 3 to 4.5 cm high.4
Retina Implant AG’s device. One of the other groups that has implanted a device in human subjects, Retina Implant AG also reported restoring visual perception in blind patients such that they could localize and recognize objects and read letters and words that were 5 to 8 cm high.5 In 2005, the group began its first clinical trial in humans, temporarily implanting its subretinal device in 11 subjects. In 2010, a second clinical trial began with the goal of implanting the chip in 60 patients. The Wills Eye Institute is the lead U.S. clinical trial site.
Retinal prostheses are in an early stage. “There have been some clear demonstrations of visual capabilities,” said Joseph F. Rizzo III, MD, professor of ophthalmology at Harvard University Medical School and director of the neuro-ophthalmology service at Massachusetts Eye and Ear. “If the goal is to be able to say that people see something after the retina is stimulated, then the goal has been met.” But Dr. Rizzo drew a distinction between the ability to identify a cup or fork in a clinical setting, after perhaps being shown such objects multiple times, and the ability to successfully navigate in a new and unstructured environment, for example, a restaurant.
Dr. Rizzo, a BRIP cofounder, continued: “We’re trying to provide enough vision to allow patients to do activities that are beyond what they could otherwise accomplish with their severely limited vision. Just demonstrating improvement isn’t sufficient. You have to justify the risk the patient takes [with surgery and long-term safety] to have an implant.”
Stefanie G. Schuman, MD, assistant professor of ophthalmology in medical retina at Duke University, agreed. Although she tells all of her patients whose vision is 20/200 or worse about clinical trials, none of them have pursued the option. Like her, they’re waiting for a device that provides the quality and type of vision that can improve their function day to day. “They want a little more of a guarantee that it will make them see better,” she said. “With the type of visual results the devices are getting, a lot of these patients would rather not go through surgery and the cost of travel.”
How They Work
A retinal prosthesis first has to capture a visual image, either with an external camera or a sensor inside the eye. Ultimately, those images provide electrical input to the visual pathway; thus, patients must have healthy optic nerves.
The Argus device uses a camera and transmitter mounted on eyeglasses, an implanted receiver, and an array of electrodes secured to interface epiretinally with retinal ganglion cells. A battery pack worn on the patient’s belt powers the system.
The camera captures images as the subject’s head moves to view objects and track movement. These images are processed by the transmitter and receiver and turned into electrical impulses on the epiretinal array. These electrical impulses are intended to stimulate the retina’s remaining cells and generate corresponding perception of patterns of light in the brain, which patients interpret as meaningful images.
The Retina Implant AG prosthesis doesn’t have an external camera. Rather, it uses a light-sensitive microchip that is surgically implanted under the retina, in the macular region where photoreceptor cells are located. The implant moves with the eye, which provides for “more natural processing of the image.”5
Aside from the subretinal microphotodiodes, the only other equipment is a power module implanted behind the ear.
Problems to Overcome
Although the prosthetic technology has come a long way since 2002, researchers have many challenges ahead if visual quality is to improve.
Placement of chip affects electrodes. Whether to place the device on or under the retina is an engineering decision, and each decision has trade-offs, said James D. Weiland, PhD, associate professor of ophthalmology and biomedical engineering at the University of Southern California. The subretinal device requires more surgical skill than the epiretinal implant, which goes through the pars plana. The subretinal Retina Implant AG prosthesis has 1,500 pixel-generating electrodes; the epiretinal Argus II has only 60. Experts agree that a minimum number of electrodes is required in order to achieve useful vision, but it’s not known for sure what that number is.
Dr. Weiland said he believes it takes somewhere between 300 and 1,000 pixels to create a usable image. But, he noted, “You can have an array with a million pixels. But if a single electrode doesn’t have enough energy to activate the retina, then really you don’t have a million-pixel array. It’s the functional pixels that matter.” He drew an analogy: You have a high-definition TV, but you’ve lost your glasses. “If you don’t have your glasses, it doesn’t matter how high the resolution of the TV is.”
Dr. Weiland continued, “We still need to understand how to get better resolution.”
Hermetics. Dr. Rizzo said that hermetics is also a great challenge. For example, the BRIP’s stimulating chip, which is inside a titanium case, has wires running to the outside, which connect to other parts of the prosthesis. BRIP is trying to develop a connecting mechanism that allows egress from the case while keeping water vapor and sodium ions from entering and damaging the electronics.
The Argus II has a hermetic package for its 60 electrodes that has allowed it to remain in place—at least in one patient—for more than four years so far.6
To date, the subretinal Retina Implant device has remained in subjects’ eyes for up to three months before explantation,6 and as a result it hasn’t been determined how long it can last in vivo.
Next Steps: Mimicking Normal Vision
In normal vision, light enters the eye and is focused on the retina. “Some parts of the retina are activated, and some are not,” Dr. Weiland said. “We’re trying to do the same thing with electricity. We’re trying to activate a small part of the retina. But we don’t know how to stimulate that precisely. We need to understand the biological side of the interface.”
Functional MRI may provide some answers. “There are many points along the way where the signal is being processed by the brain,” said Dr. Weiland. “We’d like to activate the device and then scan the brain as it is working.” He added that basic animal studies may also “give us access to the signals from the retina. Then we can stimulate a retina and see how it responds to electric stimulation.”
State of the art. “We have a longer way to go before we can deliver to people the vision that will change their lives,” Dr. Rizzo said. The current generation of devices yields images that Dr. Rizzo compares to an impressionist painting, something beautiful but “blobby.” His goal is something closer to the effect of pointillist paintings, composed of thousands of individual pixel-like points.
In the meantime, Dr. Rizzo said the quality of the devices is getting better and better, and they are generally well tolerated. “Now we have to show that there’s real benefit.”
___________________________
1 www.usc.edu/hsc/info/pr/hmm/03winter/sight.html.
2 Rizzo JF. J Neuro-Ophthalmol. 2011;31(2):160-168.
3 Humayun MS et al. Interim Performance Results from the Second Sight Argus II Retinal Prosthesis Study. Paper #2594 presented at ARVO; May 3, 2011; Fort Lauderdale, Fla.
4 Sahel JA et al. Subjects Blind From Outer Retinal Dystrophies Are Able to Consistently Read Short Sentences Using the Argus II Retinal Prosthesis System. Paper #3420 presented at ARVO; May 3, 2011; Fort Lauderdale, Fla.
5 Zrenner E et al. Proc Biol Sci. 2011;278(1711):1489-1497.
6 Weiland JD et al. Ophthalmology. 2011;118(11):2227-2237.
___________________________
Dr. Rizzo is cofounder of the Boston Retinal Implant Project. Dr. Schuman reports no related financial interests. Dr. Weiland receives research funding from Second Sight. He has no equity in the company.