Download PDF
With resounding stem cell success in the hematopoietic domain, medicine now awaits a game changer for the nervous system, particularly after a clinical trial for spinal cord injury abruptly came to a halt last year.
“The eye is leading the way,” said Henry J. Klassen, MD, PhD, associate professor of ophthalmology at the University of California, Irvine. “With some good clinical results, we may soon turn the corner. Then we’ll really be in regenerative medicine.”
Focus on RPE
More than one company is putting its money on the retina, with researchers either recruiting or preparing to recruit patients for about a dozen clinical trials.
Ease of use. Much of the current work is focused on the retinal pigment epithelium (RPE), which is ideal for translational trials, in part because it’s not difficult to turn stem cells into RPE, said Steven D. Schwartz, MD, professor of ophthalmology and chief of the retina division at the University of California, Los Angeles.
Back from the brink. “The RPE is a final common pathway of vision loss, so replacing it might provide a ubiquitous treatment for a number of blinding conditions. The hope is that RPE replacement cells may not only keep cells adjacent to them alive but also begin to rescue and rejuvenate cells that are at ‘death’s door,’” Dr. Schwartz said.
Choose Your Path
Which regenerative approach has the best claim? Which will make all the difference? One approach “doesn’t negate the potential utility” of another, said Dr. Klassen, explaining that more than one stem cell strategy may be employed, even with the same disease.
Cellular options. Pluripotent human embryonic stem cells (hESCs), which were first isolated 14 years ago, can perpetuate indefinitely, generating any cell type in the body. “They’re scalable, which is an advantage, and basic science evidence suggests their immune surface markers are modified by their environment,” said Dr. Schwartz.
Although hESCs are powerful, agreed Dr. Klassen, their proliferative potential can also raise concerns about purity and tumorigenesis.
For his part, Dr. Klassen is partial to retinal progenitor cells (RPCs), which are multipotent cells that display a vigorous, but limited, burst of proliferation. “They’re the least manipulated and are naturally programmed to do what we’re trying to achieve.”
Induced pluripotent stem cells (iPSCs), which are similar to hESCs, were first produced from adult human cells in 2007. Derived from the patient, they obviate the need for immunosuppression.
Again, purity is essential. “Our purification methods were pivotal” for the investigational new drug regulations, said Dr. Schwartz. “Early results show no signs of hyperproliferation or tumorigencity. We’re encouraged. You don’t want a teratoma growing in your eyeball.”
Will the retina benefit from adult tissue stem cells, as the cornea has? Perhaps, said Dr. Schwartz, but for now, these cells are hard to find, harvest, and grow. “Currently, they’re impossible to scale for treating a disease as prominent as macular degeneration.”
No gold standard? Although preferences for certain cell types may persist among researchers, development of a gold standard appears unlikely, said Dr. Klassen. “Strategically, it may be essential to first preserve the host tissue to the greatest extent possible. But if the degenerative situation progresses, we will need to repopulate various cellular populations, rebuilding whatever is lost. So there’s no reason to jump on one horse or the other.”
|
Retinal Rescue
|
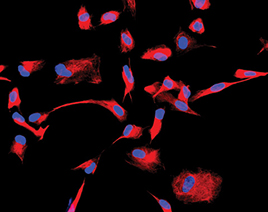 |
|
These fluorescently labeled human retinal progenitor cells (RPCs) were grown in the laboratory. The immature cytoskeletal marker nestin is labeledin red, while the nuclei appear blue.
|
First to the Clinic: hESCs
The patient numbers were minuscule, but the interest evoked was massive. Funded by Advanced Cell Technology (ACT), the first hESC clinical trial involved subretinal injection of 50,000 hESCs—differentiated to greater than 99.9 percent RPE purity—into one eye of a patient with Stargardt macular dystrophy and one eye of a patient with dry AMD. Prior to transplantation, the two patients received tacrolimus and mycophenolate mofetil in a solid-organ-transplant dosing regimen.1
Safety first. At four months, there was no sign of hyperproliferation or immunorejection, said Dr. Schwartz, the principal investigator of the trial. Functional visual improvements were also reported in both patients, “although a placebo [response] or other confounding effects could not be ruled out,” he said. “From an ophthalmic perspective, this is an early safety trial for unmet medical needs. From a regenerative medicine standpoint, it’s the flag on the moon.”
Confirming safety with subretinal injection is critical for the field, said David M. Gamm, MD, PhD, associate professor of ophthalmology and visual sciences and director of the McPherson Eye Research Institute at the University of Wisconsin, Madison.
Location, location, location. “One novel aspect of our strategy is that we’re not surgically locating the cells underneath the middle of the atrophic macula, surrounded by leagues of atrophic, long-dead tissue,” said Dr. Schwartz. “We’re transplanting the stem cell–derived RPE at the border of the atrophic zone and the still-viable retina. This is where Bruch’s membrane may be more receptive to engraftment, native RPE is available for interdigitation of the newly transplanted cells, and photoreceptors are hypothetically viable for rescue. We feel this may be a strategic and tactical advance.”
Dr. Schwartz added, “If the safety profile holds up, the next step will be to work with patients at an earlier stage of the disease. Intervening when patients still have meaningful central vision will allow us to answer efficacy questions far more reliably than in patients with very low vision.”
Looking ahead. The hESC-RPE trial has now expanded from UCLA to three other institutes in the United States and two in Great Britain. “For now, it’s all about safety,” said Dr. Schwartz. “Secondarily, are the cells taking; is there objective evidence that they are in the right place; is there any functional signal? Then, assuming safety, we’ll use what we learn from phase 1 to inform choices as we construct the next trial.”
Dishing Up a Retina?
Will researchers eventually be able to grow retinas in the lab?
Producing tissue. In 2011, Dr. Gamm and his colleagues demonstrated the ability to make optic vesicle–like structures and to isolate 3-D populations of RPCs from both hESC and iPSC cultures.1 “Then in 2012, we showed that a small percentage of isolated optic vesicle–like structures would go on to produce multilaminated retinal tissue–like structures,” he said.
Self-assembling cells. Also in 2012, Dr. Yoshiki Sasai’s lab at the RIKEN Center for Developmental Biology in Kobe, Japan, demonstrated that hESC-derived optic cups can spontaneously self-assemble and form complex retinal structures.2 “When given the proper environment, it’s quite remarkable what these cells can do on their own in a dish,” said Dr. Gamm.
___________________________
1 Meyer JS et al. Stem Cells. 2011;29(8):1206-1218.
2 Nakano T et al. Cell Stem Cell. 2012;10(6):771-785.
|
RPCs: Trial Imminent?
Dr. Klassen’s early work with rat neural progenitors prompted him to pursue the tissue-specific potential of the retina: “We had thought that there were no instructive cues left in mature mammalian nervous system tissues. But these cells seemed to have no problem at all moving in, setting up shop, reaching out to their friends and neighbors, and making connections. They had the ability to navigate and respect architectural landmarks that we thought would be gone.”
Retinal rescue. Although some of his early work had focused on replacing photoreceptors, Dr. Klassen found that RPCs used in allogenic mouse transplants could rescue the host photoreceptors. “This lowered the clinical bar. We saw the rescue of photoreceptors across the entire retina, which bodes very well. Looking at the optomotor response—the equivalent of an optokinetic nystagmus—we observed functional benefits as well.”
FDA prep. Now, after multiple allogenic and xenogenic safety studies in rats, pigs, and cats—demonstrating sterility, characterizing cells, examining karyotypes, and the like2—Dr. Klassen and his colleagues are heading to the FDA with proof-of-concept data. A preclinical trial in mice is still in progress, but the first nine months of safety data are promising, he said. “If everything goes according to plan, we hope to begin patient enrollment by the end of 2013.”
Multiple applications? “Our work is tailored to the retina, especially retinitis pigmentosa, because that’s where the risk-benefit ratio is a good fit,” said Dr. Klassen. But if it works there, he added, it might be helpful with AMD as well.
In both conditions, photoreceptors are at risk of dying. “Some are viable but not functional,” he said. “The question is, can we get them back and preserve them from a rapid-loss situation?” With regard to AMD, the preclinical benefits are tougher to document, he said, because most animals don’t have maculae. And, at this point, there is not a good dry AMD model even in monkeys.
Dr. Klassen also sees a potential benefit in retinal vascular diseases. Although this is more speculative, he said, it is not just a matter of pulling a rabbit out of a hat. “The molecules that these cells produce look like they might have a potential role in stabilizing the vasculature.”
Modeling Disease With iPSCs
Although efforts to demonstrate safety and efficacy in stem cell transplantation are important, modeling human retinal disease in a dish is already a reality, said Dr. Gamm. This involves looking at cells or tissuelike structures in isolation—not as part of a complete living organism. Still, using patient-specific iPSCs to look into the cellular mechanisms of inherited or genetic diseases holds great promise, he said.
“We know a great deal about the genes that are underlying certain retinal diseases, but oftentimes we don’t know very much with regard to the biology,” he said. “Understanding the pathophysiology of disease is key to developing and focusing treatments, so this offers us one more tool for screening pharmacologic or gene therapies, in addition to animal and cell culture models and clinical observation.”
Not all retinal diseases are amenable to in vitro modeling. For example, at this point, researchers have not been able to coax photoreceptor-like cells to form true outer segments in a dish, he said. “You have to be able to efficiently derive retinal cell types from human iPSCs, isolate them, and then manipulate and test them in culture. In some cases, it is possible to make them, but they don’t become as mature as one might like. It’s something many of us are working on.”
Remaining Challenges
A great deal of groundwork has been laid for donor cell production, said Dr. Gamm, but moving forward, countless challenges remain.
Donor cell production. This involves producing donor cells (and doing so under “good manufacturing practice” conditions), ensuring safety, and testing for desired effects, whether it be rescuing or replacing retinal cells affected by disease. “Part of our work has involved looking at the RPE cells and photoreceptor precursor cells that we produce in stem cell cultures, examining their properties, and assessing their functional capacity,” Dr. Gamm said.
Donor-host interface. Once researchers have done a respectable job on the donor cell side of things, they must determine the best time to intervene during the course of each disease of interest, the optimal delivery method, and ways to modulate the host tissue environment to promote donor cell survival and integration in the eye, Dr. Gamm said.
Windows of opportunity. Is there a sweet spot for intervention? Is it best to do cell rescue therapy first and cell replacement later? These are important questions to address. “We are putting these cells into what is often a very hostile environment,” said Dr. Gamm. “It’s not like trying to plug a new carburetor into an otherwise pristine engine. There are complex domino effects of disease that we probably will have to deal with.” For example, scarring can complicate efforts to transplant cells effectively.
Cell challenges. Depending upon the cell type, there are different hurdles to overcome. Transplantation is theoretically easier with the RPE because it’s a monolayer that doesn’t require synaptic connections, but it does need to make tight junctions and adopt proper orientation to ensure critical functions, Dr. Gamm said. “For example, proper RPE function requires an epithelial structure, which may not be achievable with bolus injections of dissociated cells, but may be possible when delivered on a preformed scaffold.”
___________________________
1 Schwartz SD et al. Lancet. 2012;379(9817):713-720.
2 Klassen H et al. Stem Cells Int. 2012 May 10. doi:10.1155/2012/460504. [Epub ahead of print.]
___________________________
Dr. Gamm reports no related financial interests. Dr. Klassen is the founder of jCyte. Dr. Schwartz has no direct financial interest in ACT, but he notes that ACT does sponsor the research.