By Xian Hui Lim, MBBS, Daniel S.W. Ting, MD, PhD, and Adrian Koh, MBBS, FRCS, MMED, FRCOphth, FAMS
Edited By: Ingrid U. Scott, MD, MPH, and Bennie Jeng, MD
Download PDF
Retinitis pigmentosa (RP) is a progressive degeneration that typically starts with involvement of the rod photoreceptors, followed by cone photoreceptors,1,2 and thus is classified as a rod-cone dystrophy.
The condition is estimated to affect 1 in 4,000 people worldwide, with variable prevalence in different populations. For example, the rate of nonsyndromic RP around Jerusalem is roughly 1 in 2,086, while 1 in 1,000 older individuals in northern China have classic RP fundus findings and functional visual loss.1
Although RP usually affects only the eyes, it can occur as part of a syndrome or as a result of systemic conditions in 20% to 30% of cases.2,3 The most common syndromic RP conditions include Usher syndrome, in which RP is accompanied by hearing difficulties and possible vestibular ataxia, and Bardet-Biedl syndrome, in which patients may have RP with concomitant intellectual disability, obesity, polydactyly, hypogonadism, and renal abnormalities.3
Abetalipoproteinemia, phytanic acid oxidase deficiency, and familial isolated vitamin E deficiency are rare syndromic RP conditions in which early initiation of treatment may prevent deterioration.3
Currently, however, there is no proven treatment to prevent or reverse deterioration of vision in other forms of RP.
Genetics
Approximately 50% to 60% of RP cases are inherited in an autosomal recessive pattern, 30% to 40% are autosomal dominant, and 5% to 15% are X-linked. Digenic, maternal mitochondrial, and other non-Mendelian inheritance accounts for the remainder.3
Heterogeneity. The genetic heterogeneity of RP is remarkable. Nonsyndromic RP is associated with 56 genes and more than 3,100 mutations.2 In syndromic RP, Usher syndrome is linked with 12 genes and Bardet-Biedl syndrome with 17 genes, with a combined total of 1,200 mutations.2 Each identified gene can be affected by different mutations, resulting in different phenotypes, while similar mutations in the same gene can cause different clinical manifestations; this complexity can present a significant barrier to diagnosis as well as to the mapping of genes to disease.2
Counseling. Genetic counseling is important in equipping patients with the knowledge of inheritance patterns and the likelihood of family members having the disease. Genetic testing is valuable, as genotypes can translate into variable phenotypes. Taking a thorough family history can help home in on the type of genetic testing needed, translating into significant cost savings for patients.4
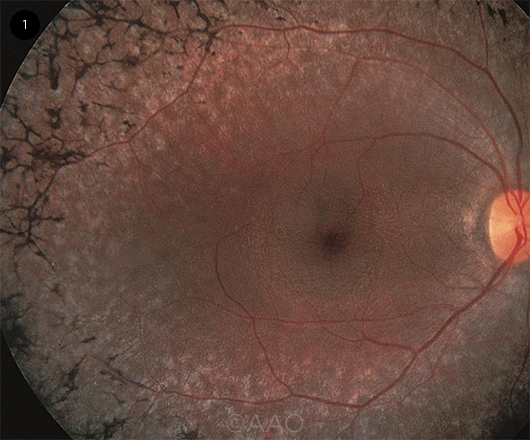 |
|
KEY FINDING. Fundus photo in RP patient shows the pathognomonic sign of bone-spicule hyperpigmentation.
|
Natural History
In 80% to 90% of patients, the loss of rod photoreceptors precedes and exceeds the loss of cone photoreceptors.5 The effect is seen in the most commonly reported onset of RP in which poor night vision occurs before impairment of color contrast and daylight visual acuity (VA).3 However, in some patients the loss of both types of photoreceptors may be comparable. A minority may have cone-rod degeneration, in which cone function declines more than rod function, with corresponding symptoms.3
Disease course. There is much interpersonal variation in the age of onset, subjective severity of symptoms, and rate of visual decline in RP.2,3 The symptoms may appear at any time between childhood and middle age, although patients frequently experience nyctalopia in their youth and lose their midperipheral vision in the early adult years.3 Subsequently, far peripheral vision is gradually lost, after which central vision constricts to become tunnel vision. Patients are often legally blind by 40 years of age owing to severely restricted visual fields and have deterioration of central vision by 60 years of age.3
Delay in presentation. Because many nighttime settings are well lit, allowing for sight with cone rather than rod photoreceptors, patients may not notice the impairment of their night vision until late in the disease.3 In addition, patients are usually able to perform everyday tasks without noticing visual field restriction until the field is reduced from the normal mean of approximately 180 degrees horizontally to 50 degrees.3 Moreover, VA can also be maintained despite a reduction of up to 90% of foveal cone photoreceptors.3 Because of these factors, patients may have a delayed presentation with RP.
Symptoms and Signs
Classic features of RP include nyctalopia, visual field loss, and reduced VA.1 Color vision and contrast sensitivity may also be impaired.3
On examination, the retina often displays pathognomonic bone-spicule hyperpigmentation in the mid- to far periphery (Fig. 1) as a result of the migration of pigment from the retinal pigment epithelium (RPE) to the neurosensory retina secondary to progressive photoreceptor loss.3 Posterior subcapsular cataracts are seen in approximately half of patients with RP.3 The optic nerve head may have a pale, waxy appearance, retinal arteries tend to be attenuated, and vitreous cells or cystoid macular edema (CME) may be observed.1-3 Macular degeneration usually occurs in advanced disease.1
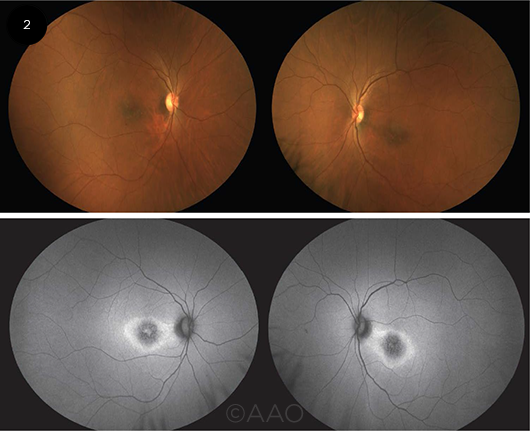 |
|
PHOTOS VS. FAF. Fundus photographs (top) and autofluorescence images (bottom) of a patient with RP caused by a heterozygous SNRNP200 mutation. The relatively subtle macular findings in the photos are more apparent on FAF: hypoautofluorescence centrally with a surrounding hyperautofluorescent ring. The FAF findings are less marked in the right eye, which corresponds to better acuity in that eye.
|
Visual Function Testing
Photoreceptor function. Electroretinograms (ERGs) are used to assess rod and cone function by measuring the timing and magnitude of electrical impulses emitted by the retina in response to light stimulation of different colors, intensities, and frequencies.3 A single dim blue flash elicits rod photoreceptor response, repeated white light flashes elicit cone photoreceptor response, and a single bright white light elicits both rod and cone photoreceptor responses.3,5 In RP, the electrical responses take longer to occur and demonstrate diminished amplitudes compared to those in eyes with normal visual function.3
Other types of ERG can also be used for evaluating patients with RP. Full-field ERG assesses the general photoreceptor function and picks up abnormalities when at least 20% of the photoreceptors in the retina are dysfunctional.5 Multifocal ERG is more specific in highlighting the distribution of impaired photoreceptor function and can reveal the characteristic pattern of photoreceptor deterioration and loss in RP.5 ERG results are thus important for diagnosing, evaluating severity, monitoring progression, and assessing treatment outcomes in RP.3
Dark adaptation threshold. Dark adaptation thresh-old testing assesses rod photoreceptor function by determining the minimum intensity of light that can be seen after the patient has been kept in a dark environment for 30 minutes. Thus, it reflects the severity of a patient’s night blindness.3
Visual fields. Visual field defects can be detected using a Goldmann perimeter or Humphrey field analyzer. Defects are usually first observed in the midperiphery, with a gradual spread peripherally. Eventually, the far peripheral fields are lost, and central fields constrict progressively in advanced RP.3
Color and contrast vision. Color vision can be tested with Ishihara plates or other equivalents and may show tritanopia in advanced RP.3 A Pelli-Robson chart may be used to evaluate for a decrease in contrast sensitivity.2
RP and Autoimmune Retinopathy: A Connection?
Antiretinal antibodies are associated with autoimmune retinopathy (AIR).1,2 Ten percent of patients with RP were found to have antiretinal antibodies in one study, while another case series found that 90% of patients with RP and CME had antiretinal antibodies.1
AIR is a rare and poorly understood group of inflammatory autoimmune diseases that comprise cancer-associated retinopathy, melanoma-associated retinopathy, and nonparaneoplastic retinopathy (npAIR).1,2 These conditions share features including antiretinal antibodies, progressive visual field defects and vision loss, and photoreceptor dysfunction.1,2 Symptoms depend on the type of photoreceptors involved and can include rapid deterioration of vision and color vision, photosensitivity, peripheral or central field defects, photopsias, and nyctalopia.2 Fundus examination tends to be normal despite reported symptoms, although iritis and vitritis have been observed, and waxy disc pallor and attenuation of retinal arterioles may develop.2
While the pathophysiology of npAIR remains undetermined, it has been suggested that a proportion of cases may arise secondary to retinal diseases such as RP with CME.2 No definitive criteria for diagnosis and management have been established. However, a recent consensus agreement suggests that essential diagnostic criteria for npAIR should include aberrant ERG results, serum antiretinal antibodies, visual loss that is not accounted for by fundus abnormalities or retinal degeneration or dystrophy, and minimal intraocular inflammation.1
___________________________
1 Fox AR et al. Am J Ophthalmol. 2016;168:183-190.
2 Braithwaite T et al. Ophthalmologica. 2012;228(3):131-142.
|
Imaging
OCT. On optical coherence tomography (OCT), the integrity of the foveal inner/outer segment (IS/OS) junction has been reported to be positively associated with VA and has been suggested to be useful in monitoring disease progression.6 In addition, the length of the IS/OS line is related to functional visual field area and mean retinal sensitivity as measured with microperimetry. Another use of OCT in RP is evaluating for CME, which can affect central vision.5
FAF. Fundus autofluorescence (FAF) is useful in assessing photoreceptor function and RPE health.6 In RP, the predominant pattern observed on FAF is a ring of hyperautofluorescence encircling the fovea. This finding has been attributed to increased lipofuscin in the RPE from accelerated degeneration of photoreceptor cells in the outer segment layer,3,6 surrounded by an area of hypoautofluorescence in the peripheral retina due to RPE atrophy from the increased metabolic stress of photoreceptor destruction.5 The border between the hyperautofluorescence and hypoautofluorescence is thought to delineate the border between functional and dysfunctional retina; correspondingly, the visual field defect commences peripherally at the border of the hyperautofluorescent ring, and this ring is generally observed to decrease in diameter as RP progresses.6
___________________________
1 Zhang Q. Asia Pac J Ophthalmol. 2016;5(4):265-271.
2 Daiger SP et al. Clin Genet. 2013;84(2):132-141.
3 Hartong DT et al. Lancet. 2006;368(9549):1795-1809.
4 National Organization for Rare Diseases. Retinitis pigmentosa. https://rarediseases.org/rare-diseases/retinitis-pigmentosa/. Accessed March 10, 2020.
5 Sorrentino FS et al. Eye (Lond). 2016;30(12):1542-1548.
6 Mitamura Y et al. J Med Invest. 2012;59(1-2):1-11.
___________________________
Dr. Lim is an ophthalmology resident at the Singapore National Eye Centre. Dr. Ting is associate consultant at Singapore National Eye Centre and assistant professor at Duke-National University Singapore. Dr. Koh is the founding partner and senior consultant at the Eye & Retina Surgeons, Camden Medical Centre, associate professor at National University Singapore, and a visiting consultant to the vitreoretinal service at the Singapore National Eye Centre. Financial disclosures: None.
___________________________
NEXT MONTH. See next month’s Ophthalmic Pearls for Part 2 of Retinitis Pigmentosa, covering research on current management and promising new therapies.