By Nathalie Pei Yu Chiam, MD, Daniel Shu Wei Ting, MD, PhD, Lee Shu Yen, FRCS(Ed), and Chong Lye Ang, FRCOphth
Edited By: Sharon Fekrat, MD, and Ingrid U. Scott, MD, MPH
Download PDF
Last month, Ophthalmic Pearls discussed risk factors, features, and examination of rhegmatogenous retinal detachments (RRD). This month, the authors continue with a discussion of RRD management.
After Dx: How to Proceed
RRDs with superior breaks that threaten the macula require urgent vitreoretinal intervention. While awaiting definitive management, patients should maintain a posture that prevents the subretinal fluid from detaching the macula.
Definitive management of RRD includes barrier laser retinopexy in select situations, pneumatic retinopexy, primary scleral buckle, primary pars plana vitrectomy (PPV) with intraocular tamponade or combined scleral buckle and vitrectomy.
Barrier laser retinopexy. This procedure is indicated for localized detachments such as subclinical retinal detachment. This is usually performed with the patient under topical anesthesia. Patients must be forewarned that, despite this treatment, the RRD may progress and require additional intervention, including surgery.
Pneumatic retinopexy. Pneumatic retinopexy is indicated for specific RRD cases, including those with break(s) confined to the superior 8 clock hours, with all breaks being confined within 2 clock hours. Contraindications include large (giant) retinal tears, proliferative vitreoretinopathy (PVR), advanced glaucoma, poor compliance with head posturing, individuals who need to travel by air, and, in some cases, pseudophakia.
The procedure, which is performed with the patient under regional anesthesia, entails transconjunctival intravitreal injection of an expansile gas bubble, plus retinopexy to the retinal breaks. In general, retinopexy is done using cryotherapy or laser photocoagulation. Transconjunctival cryopexy usually is performed before gas injection, during a single outpatient visit. For laser retinopexy, gas injection is performed initially, followed by laser photocoagulation several days later. The expansile intraocular gases include 100% sulfur hexafluoride (SF6, 0.6 mL), perfluoroethane (C2F6, 0.4 mL), and perfluoropropane (C3F8, 0.3 mL).
Reattachment can be achieved with a single pneumatic retinopexy procedure in 80% of cases and with ≥1 procedure in 98%.1
Although pneumatic retinopexy is minimally invasive, the risk of new or missed retinal breaks is greater with this procedure than with more invasive surgery such as vitrectomy or scleral buckle.2 Other possible complications include gas migration into the subretinal space, central retinal artery occlusion from elevated IOP, vitreous incarceration at the wound, accelerated cataract formation, and endophthalmitis.
Scleral buckle and pars plana vitrectomy. All breaks must be located, then treated with cryotherapy or laser retinopexy. Vitreoretinal traction must be relieved by either scleral buckling or vitrectomy. In most cases, the subretinal fluid is drained internally (via the retinal hole during vitrectomy) or externally (by scleral cut-down in primary scleral buckle surgery), if needed.
Scleral buckle surgery. This extraocular procedure should be considered for young, phakic patients with tear(s) anterior to the equator. It is not suitable for patients with a giant retinal tear or PVR.
Transscleral cryotherapy is performed around the retinal break, and the external scleral indentation from the buckle helps to support the break. The buckle-induced indentation aids in adhesion between the neurosensory retina and the retinal pigment epithelium, while relieving vitreous traction on the retina.3 Several types of scleral buckling material are available, including encirclage and segmental and radial buckles. The procedure is usually performed in the operating room while the patient is under regional anesthesia or, rarely, general anesthesia.
Surgical steps are as follows:
- 360-degree conjunctival peritomy
- Slinging recti muscles
- Localizing the break with binocular indirect ophthalmoscopy (BIO)
- Cryotherapy with or without external drainage of subretinal fluid
- Inserting the segmental and/or encircling scleral buckle
- Suturing and tightening of the buckle
- Checking of central retinal artery perfusion to determine need for anterior chamber paracentesis
- Antibiotic wash around the buckle
- Closing the conjunctiva
- Subconjunctival antibiotic and steroid injections
Intraoperative complications include scleral perforation and recti muscle trauma/slip. In cases requiring subretinal fluid drainage, the surgeon must be aware of risk for suprachoroidal hemorrhage, hypotony, and retinal incarceration at the drainage site. Postoperative complications include PVR formation, re-detachment, buckle migration/extrusion, buckle-related infections, refractive changes, ocular motility disorders, anterior segment ischemia, and glaucoma (from vortex vein or ciliary body compression).
Among suitable cases, reattachment can be achieved with a single primary scleral buckle procedure in 80% to 90%.4
Pars plana vitrectomy. PPV may be indicated for posterior retinal break, multiple breaks in different meridians, giant retinal tear, concurrent PVR, and dense vitreous hemorrhage obscuring the retinal break(s). PPV is performed in the operating room while the patient is under regional anesthesia or, rarely, general anesthesia.
Steps include:
- Creating three sclerostomy ports (for the infusion cannula, illumination probe, and vitrectomy handpiece)
- Core vitrectomy, shaving the vitreous base, and relieving any traction over the retinal break
- Using perfluorocarbon liquid to flatten the retina and displace the subretinal fluid via the original retinal break (optional step, depending on surgeon preference)
- Retinopexy around retinal breaks; laser is often used
- Fluid-air exchange
- Injecting vitreous substitute such as isoexpansive gas or silicone oil
Nonexpansile intraocular gas tamponade, such as SF6 20%, C2F6 15%, or C3F8 15%, will usually last two weeks, three weeks, and eight weeks (respectively) due to different rates of resorption. Patients should be advised about the postoperative posturing necessary to allow the buoyant vitreous substitute to tamponade the break. This posturing is maintained until most of the gas bubble has been resorbed.
If silicone oil tamponade is used, it is typically removed three to six months after surgery; in some eyes, it is retained indefinitely.
The success rate of PPV for RRD ranges from 64% to 96%, depending on the complexity of the case.5
Intraoperative complications include trauma to intraocular structures (e.g., iatrogenic retinal breaks or iatrogenic cataracts) and vitreous/retina incarceration at sclerotomy wounds. Postoperative complications may include endophthalmitis, sympathetic ophthalmia, glaucoma, and cataract.
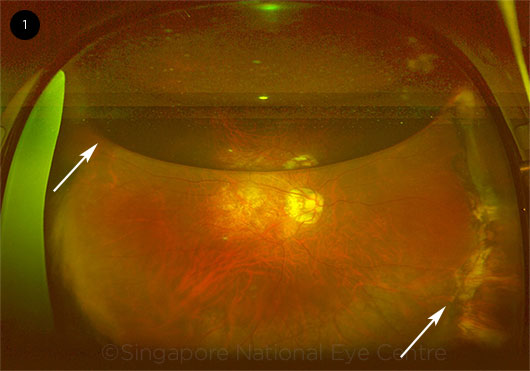
Combined scleral buckle and pars plana vitrectomy. This combination is sometimes needed for simple RRD (Fig. 1). Although most comparative studies of scleral buckle, PPV, and the combination procedure showed no significant differences in success rates for single-session surgery, a few have demonstrated that PPV alone is superior to scleral buckle alone for primary RRD.5 In a retrospective study at Singapore National Eye Centre, patients who received the combination procedure had better anatomic success rates than those who underwent PPV alone (90% vs. 80%, p < .001).6
In complicated RRD cases, combining scleral buckle and PPV can improve visualization of breaks during PPV and provide better support of the peripheral retina.
 |
|
AFTER COMBINATION SURGERY. Ultra-widefield fundus photograph of an eye that underwent scleral buckle and PPV with gas. The photograph was obtained several weeks postoperatively. A partially resorbed gas bubble is visible (left arrow), and the indent from the buckle can be seen supporting the peripheral retina (right arrow).
|
Timing of Intervention
The urgency to repair RRD depends on the status of the macula and other patient-specific characteristics. Even if the macula is on (fovea spared), urgent intervention may be necessary. When the fovea is already detached (macula-off), reattaching the retina may be less urgent. Some experts suggest that the number of days of foveal detachment may indicate the urgency of surgery. Thus, if the fovea has been detached for two days, surgery should be performed within two days.7
In a study of patients with macula-off retinal detachment, those who underwent surgery within three days of developing central vision loss had better visual outcomes postoperatively.8 However, the visual outcomes for cases in which surgical repair was delayed for 10 days did not differ significantly from outcomes for cases not surgically repaired until a month following the loss of central vision.8
Conclusion
The management of RRD requires a detailed assessment to ensure identification of all breaks. This facilitates the planning and execution of surgical intervention. Surgical treatment entails locating and sealing all breaks as well as relieving vitreous traction. Prompt intervention may produce better visual outcomes. Care should be taken to select the most appropriate procedure or procedures, with consideration given to the timing of intervention.
___________________________
1 Hilton GF, Tornambe PE. Retina. 1991;11(3):285-294.
2 Chan CK et al. Surv Ophthalmol. 2008;53(5):443-478.
3 Sullivan P. Techniques of scleral buckle. Ryan’s Retina, Vol 3. 6th ed. Philadelphia: Elsevier; 2017:1889-1915.
4 Thelen U et al. Acta Ophthalmol. 2012;90(5):481-486.
5 Young HY et al. Primary vitrectomy in rhegmatogenous retinal detachment. Ryan’s Retina, Vol 3. 6th ed. Philadelphia: Elsevier; 2017:1933-1942.
6 Wong CW et al. Retina. 2014;34(4):684-692.
7 Hassan TS et al. Ophthalmology. 2002;109(1):146-152.
8 Frings A et al. Br J Ophthalmol. 2016;100(11):1466-1469.
___________________________
Dr. Chiam is an ophthalmology resident at Singapore National Eye Centre. Dr. Ting is the surgical retinal fellow at the Singapore National Eye Centre and an assistant professor at Duke-National University Singapore (Duke-NUS) Medical School. Dr. Lee is the senior consultant, deputy head of the Surgical Retinal Department of Singapore National Eye Centre, and an adjunct associate professor at Duke-NUS. Dr. Ang is a senior consultant at the Surgical Retinal Department of Singapore National Eye Centre and a clinical professor at Duke-NUS. Financial disclosures: None.
___________________________
Acknowledgment: We thank the Ocular Imaging Department, Singapore National Eye Centre, for providing the retinal photographs in Parts 1 and 2 of this series.