Download PDF
Despite the availability of good treatment, babies continue to go blind from ROP, in large part because they have not been screened in a timely manner. “One of the main reasons is that not enough physicians are available,” said Darius M. Moshfeghi, MD, director of telemedicine at Byers Eye Institute at Stanford University. “The camera allows them to be.”
An emerging technology, store-and-forward telemedicine involves capturing medical data to be interpreted by a remote expert. It may hold special promise for retinopathy of prematurity (ROP), a condition on the rise at a time of decline in the number of adequately trained ophthalmologists able or willing to perform in-person exams.1
With better Internet access, a commercially available portable wide-angle digital retinal camera, and a dozen or so studies suggesting success in infants, telemedicine seems poised to play a larger role in the screening of ROP.1
|
Should These Eyes Be Treated?
|
 |
|
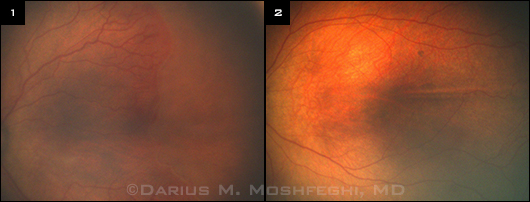
(1) Zone I, early flat neovascularization temporally with mild to moderate pre–plus disease superotemporally. Close inspection of the temporal area shows neovascularization anterior to the ridge, heralding aggressive posterior ROP requiring treatment. (2) In contrast, this eye with zone II, stage 1 ROP is low risk, with more retinal vascularization (greater zone) and no plus or pre-plus disease.
|
Growing Evidence
In 2012, the Academy published an Ophthalmic Technology Assessment (OTA) on the use of wide-angle digital retinal photography for ROP screening. It reviewed evidence from seven level I studies and three level III studies, which included 450 babies screened with telemedicine.1
“The net summary was that you can generally get the same diagnosis from a photograph as you can from an eye exam,” said OTA lead author Michael F. Chiang, MD, at Oregon Health & Science University.
SUNDROP. One of the longest-running telemedicine programs in the country is the Stanford University Network for Diagnosis of Retinopathy of Prematurity (SUNDROP), led by Dr. Moshfeghi. The singular goal of the program, he said, is to take standardized images of eyes to identify babies who need treatment. “With eight years of data from about 700 babies, we have identified—100 percent of the time—all retinopathy that requires treatment. We’ve lost no babies to blindness.”
e-ROP. A multicenter clinical trial sponsored by the National Eye Institute, e-ROP will soon add to the pool of knowledge. In October 2013, it completed enrollment of 1,284 babies from 12 neonatal intensive care units (NICUs) in the United States and Canada. “Previous studies have generally had a small number of babies and varying outcome measures,” said Graham E. Quinn, MD, MSCE, lead investigator for e-ROP, at Children’s Hospital of Philadelphia. “We needed to do a sufficiently powered study to evaluate validity, safety, feasibility, and cost-effectiveness.”
In e-ROP, trained nonphysician ROP imagers use standard protocols to capture five retinal images in each eye and upload them for remote evaluation by trained nonphysician readers to identify morphology consistent with referral-warranted ROP: zone I ROP, stage 3 or worse ROP, and/or plus disease.2 This is the first ROP study to use nonphysician readers, said Dr. Quinn. Doctors perform binocular indirect ophthalmoscopy (BIO) at the same session as images are taken, and the results from image evaluation will then be compared with results from exam.2
True gold? Other studies, such as Photo-ROP,3,4 have similarly compared photographs with BIO, and sometimes the results don’t agree. The question is, what conclusion should be drawn in case of inconsistency, especially when the photograph is right and BIO—the current gold standard—is mistaken? It’s also true that the camera may miss disease, said Dr. Moshfeghi, but this disease is almost certainly in zone III (the peripheral temporal retina), where it is of less concern because, under current guidelines, it would not trigger a treatment recommendation.
BIO is an imperfect gold standard, agreed Dr. Quinn. And, for this reason, Dr. Chiang speculates about a future gold standard that combines the two.
A Big Plus?
Telemedicine may be particularly helpful in diagnosing plus disease, one of the criteria for type 1 ROP, which warrants treatment. But identifying the tortuous and dilated vessels that define plus disease is subjective.
“When looking at a baby in person, doctors will often not agree about plus disease,” said Dr. Moshfeghi. “They will argue about where the line is and whether or not it’s crossed over into plus disease. But if you’re relying on BIO, it has to be simply ‘trust me.’ Whereas, I can support my position with images.”
However, he added, it’s also more difficult to confirm plus disease with only one set of images. It helps to see changes over time. Is it the same or different? Is the vessel more or less squiggly? “And what exactly does it mean to be squiggly or thick?” said Dr. Chiang. “We and others have been working on computer algorithms for plus disease diagnosis, to try to quantify metrics that have been subjective in the past.”
“Developing quantitative methods to confirm plus disease are certainly needed, added Dr. Quinn, “but we’re not there yet.” In addition, he said, sometimes the physician doesn’t have the luxury of waiting to compare images—and must make a diagnosis at a single moment in time. |
Complementary Roles
The camera’s role. One type of camera commonly used for ROP screening is the RetCam, a digital fiberoptic wide-angle fundus camera with a 130-degree view. The goal of this type of screening is to spot clinically significant disease that requires treatment, said Dr. Moshfeghi. The camera is able to image all of zones I and II in any baby’s eye, he said.
In-person BIO exams. According to Dr. Moshfeghi, in-person exams play an important role in two situations: to help make a treatment decision and to determine when to end acute-phase screening of ROP. Screening is generally terminated for babies who have attained mature retinal vascularization, zone III vascularization without previous zone I or II ROP, or regression of ROP. This is where BIO proves particularly helpful, he said, because the camera is not as good at capturing zone III.
Capitalize on both. “The point of telemedicine is not to replace doctors with machines,” said Dr. Chiang. “Doctors are still responsible for managing the disease, but they are doing it remotely and with fewer in-person exams.”
Meeting Resource Demands
The most obvious application of telemedicine for ROP is in overcoming geographical challenges and shortages of resources.
Shortage of ROP specialists. BIO is resource- and time-intensive. And there is a mismatch between where the babies and the physicians who treat them are located, said Dr. Moshfeghi, who is one of fewer than three dozen pediatric retina specialists in the United States. Other physicians who may examine these babies include general ophthalmologists, pediatric ophthalmologists, and retina specialists.
Making matters worse is the continuing exodus of ophthalmologists from ROP screening altogether—largely due to concerns about liability, compensation, and other financial issues. According to a 2006 Academy survey, only about half of retina specialists and pediatric ophthalmologists were willing to do ROP screening, said Dr. Chiang. “Of those, 20 percent planned to quit soon.” Telemedicine allows these dwindling resources to stretch further.
More efficient use of resources. According to current guidelines, fewer than 10 percent of babies who are screened end up needing treatment, said Dr. Quinn. “That means the serial exams of those babies snowball, and you have a huge manpower expenditure to detect a small number of babies needing treatment.”
On the other hand, telemedicine allows you to have continuous coverage with fewer resources—and you can get an immediate second opinion from anyone on Earth, said Dr. Moshfeghi. “With one click you can send it off in a HIPAA-compliant manner to world experts and say, ‘what do you think?’”
Overcoming geographic limitations. Telemedicine is a special boon to premature babies in remote rural areas or developing countries. Daniel T. Weaver, MD, a pediatric ophthalmologist in Billings, Mont., can attest to that. He was one of three pediatric ophthalmologists in Montana until 2006, when one relocated, leaving a Level 3B NICU in Great Falls without coverage for ROP. The NICU faced closure, and the hospital was threatened with a potential financial loss, he said.
Although he and the other pediatric ophthalmologist considered traveling to the babies, Montana’s size, terrain, and severe weather posed major challenges. They agreed instead to help set up a telemedicine program, modeled closely after SUNDROP but on a smaller scale. They consulted with Dr. Moshfeghi and brought in one of Stanford’s nurses to assist in training Great Falls nursing staff.
“We set specific criteria for in-person exams, and the hospital agreed to transport babies by ground or air ambulance within 24 hours for BIO examination and possible treatment,” said Dr. Weaver. In seven years, only 14 of 198 screened infants (7 percent) have been transported for further examination; of these, just 10 babies required treatment. Screened infants underwent a total of 754 RetCam examinations by the NICU staff, he said.
Some developing countries have found other ways to address their resource deficits. “One of the best ROP telemedicine programs is in India, where the need is great,” said Dr. Quinn. “It’s estimated that two babies require treatment every two hours.” Pediatric vitreoretinal specialist Anand S. Vinekar, MD, developed the program, called KIDROP. A well-trained technician takes the RetCam, carried in the back of an SUV, to the babies who need screening, and Dr. Vinekar reviews the images on his iPhone, said Dr. Quinn.
The cost factor. At around $100,000, the RetCam is not inexpensive. However, as a point of comparison, an incubator for a baby costs $75,000 to $80,000, said Dr. Moshfeghi. “And you can pay for that camera over five to seven years by amortizing costs—based on photos alone.
“In the whole scheme of things,” Dr. Moshfeghi continued, “it’s a relatively low expense to have someone provide ROP screening in the NICU to prevent blindness.”
Other Potential Benefits
Even in areas without resource shortfalls or difficult geography, telemedicine offers some benefits.
Easier on the baby. BIO, especially with scleral depression, can make premature infants unstable, said Dr. Chiang. “So sometimes we can look for only seconds, making it tough to elicit precision.” Photography, however, can be arranged around the baby’s schedule, at a time when the infant is most likely to be calm.
Helps educate parents. Sharing images with the parents may help educate them about the disease and importance of follow-up, Dr. Chiang said. “I think one of the reasons parents sometimes don’t follow up for critical outpatient ROP exams is that they don’t understand what is happening. They look at the babies and don’t feel anything is wrong because they can’t see it. When parents understand what is going on in the retina after looking at these photos, they suddenly get it.”
Allows closer scrutiny. Getting a clear reading from the bedside exam can be difficult, said Dr. Chiang. “The macula is not that well defined in premature babies,” he said, “and babies can wiggle around so much you can’t always tell whether they’re in zone I or zone II. In contrast, with a photo, you can more precisely map it out and scrutinize it to reach a diagnosis.”
For example, at first glance, the eye in Figure 1 could be mistaken as having stage 1 ROP, said Dr. Moshfeghi. “However, if one takes the time to look carefully straight temporally, one can appreciate the flat neovascularization just anterior to the ridge, heralding the onset of aggressive posterior ROP.” BIO six hours later confirmed these findings, with the eye worsening in the interval. The infant was treated with laser photocoagulation in both eyes and had excellent functional and anatomic outcomes. “The photograph allows one to study the findings at length to arrive at the appropriate diagnosis,” he said.
Objective documentation. Describing the hand-drawn images that ophthalmologists must create to record BIO findings, Dr. Moshfeghi asked, “Can you imagine someone drawing out an EKG? Why draw the retina when you can photograph it?”
Hand-drawn images are not standardized; thus, independent verification of the diagnosis may be difficult, as is following progress week to week in serial drawings, said Dr. Moshfeghi. But a series of photos can easily be laid side by side or on top of one another to compare progression, said Dr. Chiang. “That is, in essence, the beauty of telemedicine,” added Dr. Moshfeghi. Telemedicine also encourages you to systematically write down all your findings, he said, which can facilitate spotting any errors and provide a kind of belt-and-suspenders approach.
Residency education. Some ophthalmology residency programs are finding that they have too few affected infants in their clinic to provide adequate training in ROP for fellows in retina and pediatric ophthalmology, said Dr. Weaver. “If you’ve got a telemedicine system, you can image babies and use the photos to teach in a way you can’t do otherwise,” he said, “especially since exams are hard on these babies, affecting heart rates, breathing, and stress levels.”
Research. Dr. Quinn is also hopeful that standardization of images and creation of image libraries may facilitate a uniform approach to treatment trials. “You might work out the benefits of proposed treatment more quickly if you have a standard way of looking at it.”
___________________________
1 Chiang MF et al. Ophthalmology. 2012;119(6):1272-1280.
2 Telemedicine Approaches to Evaluating Acute-phase ROP (e-ROP). Identifier NCT 01264276. www.clinicaltrials.gov. Accessed Jan. 9, 2014.
3 Photographic Screening for Retinopathy of Prematurity (Photo-ROP) Cooperative Group. Retina. 2008;28(3) suppl:S47-54.
4 Scott KE et al. Ophthalmology. 2008;115(7):1222-1228.e3.
___________________________
Michael F. Chiang, MD, is professor of ophthalmology and medical informatics and clinical epidemiology at Oregon Health & Science University, in Portland. Financial disclosure: Is an unpaid member of the scientific advisory board for Clarity Medical Systems, the manufacturer of RetCam.
Darius M. Moshfeghi, MD, is director of telemedicine at Byers Eye Institute and associate professor of ophthalmology at Stanford University Medical Center, in Palo Alto, Calif. Financial disclosure: Has equity in Visunex Medical Systems.
Graham E. Quinn, MD, MCSE, is professor of ophthalmology at Children’s Hospital of Philadelphia. Financial disclosure: Has received NEI support for e-ROP.
Daniel T. Weaver, MD, is a pediatric ophthalmologist in Billings, Mont. Financial disclosure: None.