Download PDF
This month, News in Review highlights selected papers from the original papers sessions at AAO 2020 Virtual. Each was chosen by the session chairs because it presents important news or illustrates a trend in the field. For details of presentation times, check the Virtual Meeting Guide at either aao.org/2020 or in the virtual meeting platform.
In a phase 1/2A clinical trial, cultured retinal pigment epithelial (RPE) cells were successfully transplanted into patients with advanced dry age-related macular degeneration (AMD).1
Implanted subretinally, the human embryonic stem cell–derived RPE cells (OpRegen, Lineage Cell Therapeutics) were well tolerated. The initial cohorts showed evidence of improved structural changes during up to four years of follow-up, said Christopher D. Riemann, MD, at the University of Cincinnati and the Cincinnati Eye Institute.
“We’ve done 18 patients so far, and the OpRegen cells have a clear safety signal. We’ve also got anatomic [evidence of] slower progression of the geographic atrophy, resolution of drusen, and restoration of outer retinal and RPE anatomy,” Dr. Riemann said. “Both structural and functional data suggest a substantive and important potential signal of efficacy.”
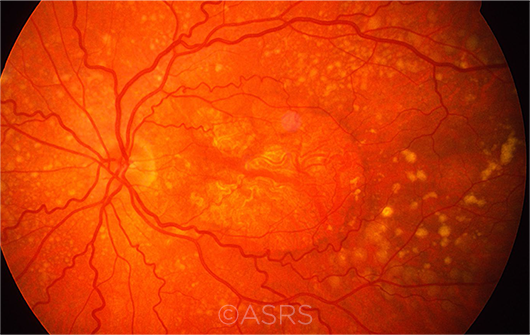 |
DRY AMD. Multiple drusen, RPE changes, and geographic atrophy are evident in this patient with dry AMD. This image was originally published in the ASRS Retina Image Bank. Henry J. Kaplan, MD, and Niloofar Piri, MD. Dry Age-Related Macular Degeneration. Retina Image Bank. 2013; Image Number 5468. © The American Society of Retina Specialists.
|
Initial safety trial. In the first 15 eyes treated, pars plana vitrectomy and retinotomy were performed in order to inject a suspension of 50,000 to 200,000 cells subretinally, Dr. Riemann said. The stem cells used in the trial came from an ethically sourced, federally approved, 20-year-old cell line derived from embryos that were abandoned following in vitro fertilization.
With regard to adverse events, 13 of the 15 eyes developed macular fibrosis or macular pucker. Serious ocular adverse events included one severe epiretinal membrane (ERM) and one retinal detachment, both of which required surgical repair, Dr. Riemann said.
Persistent subretinal pigmentation was visible in most of these eyes in the area where the cells were implanted. “We think this actually represents the sustained cells that have survived,” he said.
Next steps. In the ongoing trial’s latest study cohort, which comprises 12 patients, those with better visual acuity (20/64 to 20/200) are being enrolled, Dr. Riemann said. “We’re looking at eyes that have better vision [than did those in the safety trial], in hopes of picking up on some sort of improved visual acuity signal after treatment,” he said. Patients in the safety trial had advanced AMD and were legally blind.
The investigators also are testing the use of a new flexible cannula infuser—Orbit SDS (Gyroscope Therapeutics)—that they hope will reduce the risk of procedural complications by eliminating the need for vitrectomy and retinotomy. Dr. Riemann said the researchers have already performed three OpRegen transplants using the Orbit SDS with excellent results and no ERM formation.
He added that the group is excited by initial indications that the nonvitrectomy approach may have solved the ERM issue seen in the initial cohort. The group hopes to have completed several procedures with the device by the time of AAO 2020 Virtual to corroborate this finding.
“The first thing we have to figure out is whether implanting these RPE cells really works as well as the early data may suggest,” he said. “Then we would want to ask, ‘If one bleb is good, are two or more blebs better?’ Because one of the exciting things is that, in animals, I’ve personally been able to create up to nine blebs in one eye with a single incision and a single Orbit SDS device.”
—Linda Roach
___________________________
1 Riemann CD et al. Phase 1/2a study of subretinally transplanted human embryonic stem cell–derived RPE cells in advanced dry-form AMD patients. Presented at: AAO 2020 Virtual; Nov. 13-15, 2020.
___________________________
Relevant financial disclosures—Dr. Riemann: BioTime/Lineage: C,S; Gyroscope Therapeutics: C,S; Janssen/Johnson & Johnson: C; Orbit Biomedical: C.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Nakazawa Canon: S; Daiichi-Sankyo: S; Kowa: S; Nidek: S; Rhoto Pharmaceutical: S; Santen: C,L,S; Senju Pharmaceutical: C,L,S: Tomey: S; Topcon: S; Wakamoto Pharmaceutical: S.
Dr. Riemann AGTC: S; Alcon: C,L,S; Alimera: C,LS; Alimera Deutschland: C,L; Allergan: L,S; Aniridia Foundation International: C; Animal Eye Institute: C; Aerpio: S; Bausch + Lomb/Valeant: C,L; BioTime/Lineage: C,S; BMC/Eyetube: C; Chengdu Kanghong: S; CSTLII: L; Chruman Research: O; Clearside: S; Clovernook Center for the Blind and Visually Impaired: C; CVP (CEI Vision Partners): O; Digital Surgery Systems: O; Gore: C; Genentech/Roche: S; Gyroscope: C, S; Haag-Streit: C; Haag-Streit Surgical: C; Haag-Streit USA: C,L,P; HumanOptics: C; IamC2: C,P; iVeena: C,O; Janssen /Johnson & Johnson: C,P,S; Kaleidoscope Engineering: C,P; Lowy-MacTel Registry: S; Med One: C,P; Macor Industries: O; Northmark Pharmacy: O; Neurotech: S; Nightstar/Biogen: S; Novartis: S; Notal Vision: C,S; Novartis: L; Opthotech/Iveric: S; Orbit BioMedical: C; Regeneron: L,S; Reliance Industries: C,L,P; SalutarisMD: C,L; Spark: S; TrueVision: C,L,P; VEO: O; Vortex Surgical: C,O,P.
Dr. Shousha NEI: S; Research to Prevent Blindness: S; Resolve Ophthalmics: O. Related patents and PCT are owned by the University of Miami and licensed to Resolve Ophthalmics.
Dr. Yu-Wai-Man GenSight Biologics: C.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|
More from this month’s News in Review