By Annie Stuart, Contributing Writer, interviewing Steven T. Bailey, MD, Ninel Z. Gregori, MD, Christine N. Kay, MD, and Krista Soto
Download PDF
Just weeks after Caspian Soto’s birth, his parents started noticing something was awry: Their baby stared constantly at lights but avoided making eye contact. “We were new parents and weren’t sure how concerned we should be,” said his mother, Krista Soto. Then his eyes began to roll up and down, and his parents’ worry increased.
After an emergency evaluation ruled out a tumor, an electroretinogram later spotted the telltale signs of Leber congenital amaurosis (LCA). Genetic testing confirmed that both parents carried a copy of a mutation in the RPE65 gene and that Caspian was deficient in both copies. Caspian officially joined the 1,000 to 2,000 Americans with RPE65 mutation–associated retinal dystrophy.1 Without treatment, his prognosis was dim.
Fortunately, Caspian was a candidate for Luxturna (voretigene neparvovec-rzyl, Spark Therapeutics), approved in December 2017 for both LCA and early-onset retinitis pigmentosa (RP). In the fall of 2018, at the age of 4, he became one of the youngest patients to be treated with Luxturna, the first FDA-approved gene therapy for a genetic disease.
About two weeks after Luxturna treatment in the second eye, Caspian’s parents began noticing some surprising changes in his ability to navigate his environment. His mother took him to a nearby children’s museum to “test his vision.” Together, they walked into an exhibit where LED stars dotted the ceiling. “He was so excited because he’d never been able to see anything like it,” said Ms. Soto. “For an hour, we just lay on the floor together and looked up.”
How Luxturna Works
Approved for patients 12 months and older, Luxturna is an adeno-associated virus vector-based gene therapy that delivers a normal copy of the RPE65 gene under the retina, said Ninel Z. Gregori, MD, at Bascom Palmer Eye Institute in Miami. The gene provides instructions for making an enzyme essential for normal vision, allowing retinal cells to function more normally.
“We don’t treat the entire retina,” said Steven T. Bailey, MD, one of the surgeons who treated Caspian at the Oregon Health & Science University (OHSU) Casey Eye Institute in Portland. “But we try to shore up central areas, where the treatment can be most useful.”
Researchers hypothesize that earlier treatment is better because the retina is likely to have less severe damage, said Dr. Bailey. In phase 1 Luxturna trials, the final level of visual sensitivity was significantly better in 8- to 11-year-olds compared with 19- to 44-year-olds, said Dr. Gregori. “But in the phase 3 trial, even the most advanced patients had some improvement in vision.”2,3
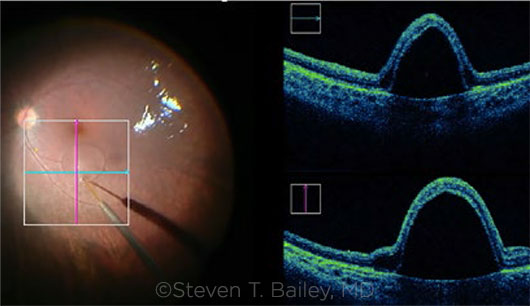 |
|
IN THE OR. Subretinal delivery of voretigene with bleb visible with intraoperative video (left) and intraoperative OCT (right).
|
Before the Procedure
According to Spark Therapeutics, more than 35 patients have received treatment since FDA approval. Currently, the surgeries are done at only seven ocular gene therapy treatment centers, using a well-defined Spark protocol, said Dr. Gregori. The first step is to identify the best surgical candidates.
Confirm the diagnosis. “Other inherited retinal diseases [IRDs] can have a similar phenotype,” said Dr. Bailey. “So we need to ensure that we’re targeting the right disease with this gene therapy. Genetic testing confirms that the patient is deficient in both copies of the RPE65 gene.”
Rule out poor candidates. It’s also essential to select only patients who have viable retinal cells. “We use optical coherence tomography [OCT] to assess for viable cells during the patient selection process,” said Dr. Gregori.
Arrange approval. “The manufacturer has a team of liaisons who help physicians communicate with patients, billing departments, and insurers to achieve approval,” said Dr. Gregori, “but it’s not an instantaneous approval process.” The $850,000 price tag might have something to do with this.
Ms. Soto’s first question was: How do we raise a million dollars? “In my wildest dreams, I never anticipated it would be covered by insurance,” she said. And up until a few days before surgery, she didn’t know what their out-of-pocket fee would be. In the end, their insurer covered most of the cost, and Spark covered the rest.
Begin steroids. Because injection of the virus puts the eye at risk for inflammation, patients are started on oral prednisone three days before surgery—21 days in total, said Dr. Gregori. Local corticosteroids are also used at the time of and after surgery.
The Procedure
The patient’s eyes are treated on separate days, with a recommended minimum interval of six days. On the day of surgery, the pharmacy prepares two sterile syringes of the drug, said Dr. Gregori.
Choosing anesthesia. Depending on the patient, the procedure is done under general anesthesia or local anesthesia with IV sedation, said Dr. Gregori.
Visualize the vitreous. Although the vitrectomy has been tolerated quite well in these patients, surgeons have made certain alterations to ensure best outcomes, said Dr. Bailey. “For example, I’ve found that using a dilute Kenalog solution is useful for visualizing the vitreous and ensuring that we’ve successfully induced a posterior vitreous detachment.”
Gently remove the vitreous. “With a 23- or 25-gauge vitrectomy, we remove the vitreous in a standard fashion,” said Dr. Gregori. “Once we separate the gel from the retina, we’re very cautious that we don’t cause peripheral breaks or detachments when we remove the vitreous. Elevating the vitreous off the macula at the proposed injection site allows the needle to penetrate the retina without being caught on the vitreous.” Removal of the sticky peripheral vitreous can be challenging in these eyes, she added, explaining that it is sometimes preferable to leave it, rather than doing a full vitrectomy and risking an iatrogenic retinal break.
Dr. Bailey emphasized that inspecting for any retinal breaks should not wait until the end of the procedure as with standard vitrectomies. “We perform scleral indentation to look for peripheral retinal breaks prior to the subretinal delivery of Luxturna. Because gene product in the vitreous cavity poses the risk of an inflammatory response, the idea is to limit ocular manipulations that may result in gene product escaping the subretinal space and entering the vitreous cavity.”
Injection site and blebs. “Avoiding vessels, we go along the major arcade, but we must inject at least 2 mm from the fovea,” said Dr. Gregori. “You can do this in one of two ways: Either inject Luxturna directly without elevating the retina, or first elevate the retina with a small subretinal balanced salt solution [BSS] bleb and then inject Luxturna into that space.”
The second of these options is beneficial in two ways, said Dr. Bailey. “You’re less likely to inject Luxturna into the vitreous cavity during initial bleb formation, and you can confirm the bleb is extending toward the fovea prior to injection. If the bleb moves away from the fovea, the surgeon can stop the injection and select one or more alternative sites to ensure the entire macula is treated,” he said.
Observe with OCT. Dr. Gregori and Janet L. Davis, MD, pioneered the use of intraoperative OCT during a choroideremia gene therapy trial a few years ago. Now, surgeons use intraoperative OCT during Luxturna surgeries. (View a video from Dr. Gregori and Dr. Davis, “OCT-Assisted Delivery of Luxturna,” below.)
With OCT in the OR, said Dr. Gregori, “we’re able to confirm that we’re injecting into the subretinal, rather than suprachoroidal, space. More important, the macula stretches with injection of this large volume of medicine, putting it at risk of a macular hole and loss of the virus into the vitreous cavity. We can observe any overstretching, wait a few minutes while the fluid is absorbed, and then inject more. Or we can form a second bleb to cover the seeing area, watching to confirm that a hole has not formed.”
Intraoperative OCT also allows the surgeon to see how much pressure he or she is applying to the retina with the subretinal cannula during initial bleb formation, said Dr. Bailey.
Injection: manual or machine. In the Luxturna clinical trials, the surgeon had a surgical assistant manually inject 300 mL of the medicine, said Dr. Bailey. “We switched to a foot pedal delivery device because we found it can deliver the product in a slower, more controlled manner.” With either method, the surgical assistant must give feedback to the main surgeon about the volume of medicine that has been injected, said Dr. Gregori. She added that both methods have their advantages, and surgeons may decide which they prefer.
Do an air-fluid exchange. An air-fluid exchange is recommended to remove any gene product that may be in the vitreous cavity to reduce the risk of an inflammatory response, said Dr. Bailey. “I have an assistant aim the infusion line more peripherally, not in the direction of the bleb,” he said. “Otherwise, pressure from the infusion line may push Luxturna out of the retinotomy.”
After the procedure, patients should avoid airplane travel until the air is reduced to 10% or less, which may take up to two weeks in eyes with retinal degeneration, said Dr. Gregori.
After the Procedure
Surgeons see these patients the first day, week, and month after surgery, at which point they are usually sent back to the referring retina specialist, said Christine N. Kay, MD. She’s a vitreoretinal specialist in Gainesville, Florida, who has sent three patients to Dr. Gregori and colleagues at Bascom Palmer. She sees these patients as needed postoperatively, typically right after they are released from their treatment center and one month, three months, and six months after treatment. “Spark also requests that patients return to the surgical treatment center at six months for repeat outcomes testing,” she said.
Tests and monitoring. The first postoperative visit includes checking vision and intraoperative pressure and looking for inflammation, said Dr. Bailey. “With subsequent visits, we use OCT to make sure all subretinal fluid has been absorbed and to assess the retinal anatomy.” Subsequent visits may include repeat visual fields and electroretinograms to assess the treatment effect, he said.
Potential complications. Patients continue with postop oral prednisone and corticosteroids drops on a relatively rapid taper over several weeks, said Dr. Bailey, and cases of inflammation have been minor so far. “As with any surgery, we worry about retinal detachment,” he said. “We may assess the peripheral retina with ultrasound if an indirect ophthalmoscopy exam is too challenging to do in a young child.” If retinal holes are visible, added Dr. Gregori, it’s important to laser those right away.
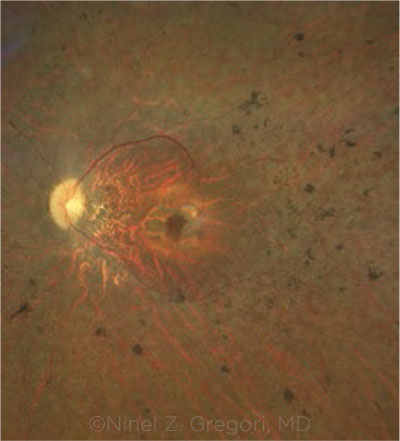 |
|
TREATED EYE. Fundus photograph of a patient with biallelic RPE65 mutations who received voretigene therapy in both eyes.
|
Patients’ Quality of Life
“I can’t even describe how Caspian’s life has changed,” said Ms. Soto, explaining that he started preschool a couple of weeks after his treatment. “I no longer felt scared that he wouldn’t be able to see the classroom space and be ostracized because of it. I didn’t worry that he would feel ‘othered’ because of his headlamp [which he used to rely on before treatment].”
A time of transition. She hastened to add that Caspian still faces obstacles. For example, being reintroduced to social situations with improved vision has brought its own set of challenges, such as learning to read facial cues. At first, Caspian was scared about the adjustment, and he balked at letting go of his headlamp and walking cane.
“Although patients are often much happier, there are many adjustments that come along with seeing better, such as being able to stay out later at night to play with peers and other social or behavioral considerations,” said Dr. Kay. “It’s important to help the patient and family navigate that process.”
Visual sensitivity. Two patients Dr. Kay has seen postoperatively have experienced dramatic improvements in visual sensitivity. “Within two weeks of surgery, the 10-year-old had significant improvement in his ability to navigate in dimly lit rooms, play outside at night, and ride a bike home in the dark,” said Dr. Kay. “Easter eggs were brighter, and he saw a rainbow for the first time.” Although the patient’s visual function subjectively improved overall—indeed, he had objective improvement in visual acuity in one eye—there was a slight decline postoperatively in visual acuity in the nondominant eye (possibly due to foveal detachment). However, the patient is unaware of this.
Visual fields. The second patient that Dr. Kay referred to Bascom Palmer—a 17-year-old with a milder phenotype of RPE65-associated LCA—experienced a dramatic improvement in his visual fields with a return of one isopter of light. Dr. Gregori considers the boy’s results the best of the patients she’s treated so far. “Even his central acuity function improved, which is interesting since foveal detachment was avoided in this patient, and the cone cells rely on Müller cells, not just retinal pigment epithelial cells,” she said. “The enhanced retinal milieu may improve the function of the cones as well.”
Long-term prognosis? “We have about three years of data proving sustained responses using the trials’ outcome measures,” said Dr. Kay. Despite improvement in visual function after this gene therapy, however, photoreceptor degeneration continues at about the same rate as the natural history, said Dr. Gregori. “The question is: What happens later on? How long do the cells continue making this protein? Will we need to reinject at some point?”
Ms. Soto said that unknowns like these are definitely the most difficult part of the process. Still, she says she’s incredibly grateful that her child’s surgeons fully prepared her to have realistic expectations. “The journey doesn’t end here, but there is so much exciting stuff happening in this field,” she said. “It’s pretty amazing.”
Testing, Testing
“Considering the demographics and epidemiology, I suspect that we haven’t identified all the patients who could benefit from this treatment,” said Dr. Kay.
Find unidentified patients. She emphasized that IRD specialists need to look through their databases to find LCA patients who haven’t yet received genetic testing. “It’s also important not to neglect early-onset RP,” she said. “Someone previously diagnosed with RP could turn out to have the RPE65 gene mutation.” She added that clinicians need to ask patients, “At which age did your vision loss begin? Did you have nystagmus as a baby?”
Testing may be free. If a patient reports onset of LCA or RP symptoms (including night blindness or visual field loss) prior to the age of 18, Dr. Kay believes they should absolutely have genetic testing to rule out RPE65-associated degeneration. Spark is still offering free single-gene testing to rule out RPE65, she said. “So there’s no reason for any patient with a diagnosis of LCA or RP—no matter the age of onset—to not have this single-gene mutation ruled out.”
Alternatively, she said, the clinician might be able to coordinate a free 266-gene panel like that offered by Foundation Fighting Blindness’ My Retina Tracker Registry through Blueprint Genetics. “But if that’s not possible, at least rule out RPE65 in these patients.”
Other IRDs. “Clinicians also need to refer patients with other IRDs for genetic testing,” said Dr. Gregori. “It’s no longer sufficient to just say, ‘You have Stargardt disease or choroideremia.’ Patients need to know their specific molecular diagnosis. Other therapies are coming down the pike, and patients may be eligible for clinical trials. Now is a great time to sit down and create a cheat sheet about where to refer specific patients for genetic testing, counseling, or treatment.”
|
___________________________
1 Shaberman B. Hum Gene Ther. 2017;28(12):t1118-1121.
2 Bennett J et al. Lancet. 2016;388(10045):661-672.
3 Russell et al. Lancet. 2017;390(10097):849-860.
___________________________
Dr. Bailey is associate professor of ophthalmology and a vitreoretinal specialist at Oregon Health & Science University Casey Eye Institute in Portland. Relevant financial disclosures: None.
Dr. Gregori is associate professor of clinical ophthalmology at Bascom Palmer Eye Institute at the University of Miami Health System and chief of the ophthalmology section at Miami Veterans Affairs Medical Center in Miami. Relevant financial disclosures: None.
Dr. Kay is a vitreoretinal specialist at Vitreoretinal Associates in Gainesville, Fla. Relevant financial disclosures: Spark Therapeutics: C; Foundation Fighting Blindness: S.
Ms. Soto is the mother of Caspian Soto, a patient at OHSU Casey Eye Institute in Portland. Relevant financial disclosures: None.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Bailey None.
Dr. Gregori Astellas Institute for Regenerative Medicine: C; Nightstar Therapeutics: S.
Dr. Kay Achromacorp: S; AGTC: S,C; Alkeus: S; Astellas: C; Foundation Fighting Blindness: S; Nightstar Therapeutics: S, C; Ophthotech: S; Novartis: C, Sanofi: C; Second Sight: C; Spark Therapeutics: C.
Ms. Soto None.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|