Download PDF
Balancing the benefits and risks of steroids is vital to optimizing ocular health, and each patient’s circumstances must be factored into the treatment equation. As the debate persists over when and how to use ocular steroids, four experts share insight on this topic and offer tips to achieve success with these powerful agents.
No one is allowed to die or go blind without a trial of steroids!” So goes the tongue-in-cheek saying among neuro-ophthalmologists, according to Deborah I. Friedman, MD, MPH, professor of neurology and neurotherapeutics and ophthalmology at University of Texas Southwestern Medical School in Dallas. Dr. Friedman and her colleagues rely on corticosteroids as first-line therapy for every inflammatory condition they treat. In many cases, the steroids are vision saving. The importance of ocular steroids to all of ophthalmology cannot be overstated. For more than 60 years, nothing has matched their effectiveness as fast-acting anti-inflammatory agents.1 If they didn't have adverse effects, steroids would be the only anti-inflammatory agents we would ever need,” said John D. Sheppard Jr., MD, MMSc, professor of ophthalmology, microbiology, and immunology and clinical director of the Lee Center for Ocular Pharmacology at Eastern Virginia School of Medicine in Norfolk. Despite the fact that sequelae of uncontrolled inflammation are irreversible, many clinicians overlook corticosteroid therapy because of their fear of side effects. “Far more harm has come from withholding steroids than from using them!” said Dr. Sheppard. Unlike in the past, physicians now have numerous steroid options; more types, various strengths and combinations, and multiple delivery routes are available. “There's still this notion out there that all steroids are the same, and it's just not true,” said Lawrence S. Morse, MD, PhD, professor of ophthalmology and vitreoretinal fellowship director of retina services at the University of California Davis School of Medicine in Sacramento. “Differences in the structure of each steroid affect their clinical and biological profiles. Clinicians need to be familiar with the profiles of each steroid they use so that they can choose the best one for each patient.
Mechanisms of Action
Understanding exactly how corticosteroids work is an active field of research. “We now know that steroids have widespread actions that affect gene expression pathways involving not only inflammation but also angiogenesis, oxidative stress, and apoptosis,” said Dr. Morse.
Dr. Sheppard explained that, specifically, steroids disrupt the inflammatory cascade by immobilizing arachidonic acid, downregulating multiple cytokine pathways including the vascular endothelial growth factor (VEGF) pathway, stabilizing cell membranes and mast cell granules, inhibiting leukocyte interaction, and slowing diapedesis.
Precautions
Ocular steroids are potent and relatively inexpensive, but their side effects are considerable.
TOPICAL, INJECTION, AND SUSTAINED-RELEASE DELIVERY ROUTES. All of these can lead to cataracts, glaucoma, secondary infection, or delayed healing.2,3 Typically, these adverse effects are manageable; so when a patient has a vision-threatening condition, steroids are warranted. Prophylaxis for secondary infections and surface support for delayed healing can reduce those side effects, and intraocular pressure (IOP) can be closely monitored and controlled with IOP-lowering medication.2,3 If glaucoma or cataracts occur, they can be treated successfully with surgery.
“It’s the less severe conditions where the question of whether to use steroids is harder to answer,” said cornea and external disease specialist Stephen D. McLeod, MD, professor and chairman of ophthalmology at the University of California, San Francisco (UCSF). No one wants to create a more serious problem than the initial condition.
SYSTEMIC STEROID THERAPY. Systemic use of steroids may lead to diabetes, osteoporosis, hypertension, gastritis, depression, insomnia, weight gain, facial distortion, aseptic necrosis of the hip, or skin thinning.1
When steroids are not essential, use another approach! That’s the bottom line, according to most experts.
Broad Use
The most common use of steroids in ophthalmology is to control postoperative inflammation. Steroids also are integral to treating conditions of immune hyperreactivity (e.g., noninfectious uveitis, graft rejection, allergic disorders such as atopic or vernal keratoconjunctivitis) and certain diseases that have both immune and infectious components (e.g., bacterial corneal ulcers).1 Moreover, steroids are key to damage control following ocular injuries.
Tenets of Steroid Therapy
HIT HARD, HIT FAST. “One of the biggest problems we see in a referral practice is undertreatment,” said Dr. Sheppard. “Many doctors are reluctant to prescribe adequate corticosteroid dosages because of fears of side effects.” Paradoxically, this leads to protracted steroid use at higher-than-acceptable doses because of failure to gain complete control over the inflammation, making it difficult to taper the steroids.
Contrary to what was taught years ago, long-term moderate dosing of a steroid is more likely to result in a cataract than is initial treatment with high doses of a strong steroid that is tapered and switched to a lower-strength steroid.4 According to Dr. Sheppard, the best approach is to use the most potent steroid as quickly as possible, then taper to a lower-strength steroid for ongoing management. He added, “This improves compliance and lowers the overall dosage, cost, and exposure to the medication’s preservative.”
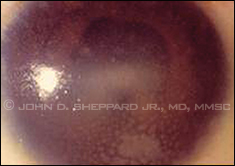 |
Acute granulomatous sarcoid uveitis
with mutton-fat keratic precipitates. |
DON’T TAPER TOO QUICKLY. Wait until the inflammation is completely controlled before tapering, Dr. Sheppard emphasized. Tapering when the eye is just starting to improve or stabilize may prolong the inflammation and the therapy. “There should be zero tolerance for cells, flare, keratic precipitates, injection, macular edema, or any other sign of inflammation,” said Dr. Sheppard. “Our longer-term goal is either to completely eliminate steroid therapy or to find the absolute minimum maintenance dose to avoid relapses.”
Dr. McLeod has an easy way to make sure that he isn’t tapering too quickly. He learned it from Todd P. Margolis, MD, PhD, professor of ophthalmology at UCSF and director of the Francis I. Proctor Foundation in San Francisco. “Todd’s trick is to ask the patient what happens if he or she misses a dose. If the patient says, ‘Nothing really bad happens; I just pick up where I left off,’ then you know that the patient is probably ready to taper. If the patient says, ‘Symptoms x and y come back,’ then it’s too soon to taper.”
INDIVIDUALIZE THE TREATMENT. Every patient is different. Dr. Sheppard noted that “a young patient often has more inflammation than an older patient; a patient who has had previous surgery and/or has existing inflammatory disease needs a lot more drug than a patient with none of those risk factors.” It’s all about tailoring the specific steroid molecule, dose (concentration), frequency, type, and delivery route to the patient’s individual needs. “That’s where the challenge is, and that’s what they pay us for—to find the best solution for each patient,” Dr. Sheppard said.
SWITCH TO STEROID-SPARING AGENTS FOR SYSTEMIC MAINTENANCE THERAPY. Systemic steroids are not a long-term option; they are appropriate only for induction therapy, said Dr. Sheppard. For chronic inflammatory conditions that require long-term maintenance therapy, corticosteroid tapering usually can be accomplished by adding an immunomodulatory agent, either a traditional drug such as methotrexate or a newer biologic agent such as infliximab. With immunomodulatory drugs, it’s prudent to team up with the patient’s internist or rheumatologist.
Long-term intravitreal implants are now a safer steroid option than systemic therapy for some chronic conditions. The implants eliminate systemic absorption and related toxicity.1 (See the “Uveitis Key Points” section for more information.)
MONITOR FOR POTENTIAL SIDE EFFECTS. “The larger the optic nerve cup and the worse the visual field, the more careful we must be in the administration of steroids,” said Dr. Sheppard. “The more potent the steroid, the more frequently you’ll need to check the patient’s [intraocular] pressure.” During steroid therapy, Dr. Sheppard usually sees patients at intervals of two to five weeks, but he sees those with significant optic nerve disease weekly in the early stages of treatment.
Preoperative and Postoperative Use
Despite the advances in surgical techniques, most patients will have some degree of inflammation after ophthalmic surgery.5 “Some patients are more susceptible than others, and there’s no way to precisely predict severity,” said Dr. Sheppard. “Therefore, all patients need inflammatory control after surgery.” Undertreatment, delayed treatment, or lack of treatment for inflammation can lead to decreased visual acuity, increased pain and discomfort, photophobia, corneal edema, and glaucoma.6
There are several hyperinflammatory reactions to surgery that require aggressive steroid therapy, said Dr. Sheppard. One of these is diffuse lamellar keratitis (DLK; aka “sands of the Sahara”) following LASIK surgery. Another is toxic anterior segment syndrome, a serious phenomenon that can occur after cataract surgery.
In addition to strict adherence to the basic tenets of steroid therapy (described above), Dr. Sheppard incorporates the following clinical pearls into his practice.
PREOPERATIVE PROPHYLAXIS. “I’m a strong advocate of starting steroid drops a day or two before surgery,” said Dr. Sheppard. “You want to prepare your ‘normal’ patients for the surgery by downregulating the activity of the inflammatory cascade, and it takes about a day or so for the steroid to kick in.” This will reduce the patient’s ability to muster a strong inflammatory response to surgery.
SYNERGY WITH TOPICAL NSAIDS. Nonsteroidal anti-inflammatory drugs (NSAIDs) and corticosteroids can work synergistically. “At the risk of oversimplifying, you prescribe steroid drops for chronic pain, anterior segment inflammation, and ocular surface inflammation,” said Dr. Sheppard. “You use NSAIDs for macular-thickness control, photophobia, and immediate pain relief.”
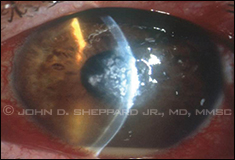 |
| Axial streptococcal hypopyon keratitis. |
QUIET EYES PRIOR TO SURGERY. For elective surgery, Dr. Sheppard strongly advises that the eye be totally quiet for three months beforehand (six months for children) whenever possible. This means that ocular surface disease (e.g., dry eye, blepharitis, allergic conjunctivitis, tarsitis), anterior segment inflammation, and macular disease should be controlled optimally. Examples of macular disease include cystoid macular edema, diabetic maculopathy, epiretinal membrane, and lamellar macular hole.
CATARACT RISK MAY BE THE LESSER EVIL. A steroid-induced cataract is preferable to irreversible ulcer cicatricial damage such as leukoma, endothelial depletion, synechiae, trabecular insufficiency, ciliary body fibrosis, and maculopathy, said Dr. Sheppard. “Everyone is so paranoid about giving a patient a steroid cataract, but inflammation can be far more dangerous. If a patient takes so much steroid that he develops a cataract, then so be it. A cataract can be removed. It’s our best operation; the prognosis is excellent. Just don’t remove the cataract until the inflammation has been fully controlled for three months.”
OTHER MEDICATIONS. Oral prophylaxis for toxoplasmosis and herpes simplex virus is imperative for preventing relapses after surgery. It’s also essential to avoid medications and ingredients that exacerbate inflammation (e.g., prostaglandin analogues) or ocular surface disease (e.g., preservatives, topical beta-blockers, and systemic antihistamines, diuretics, and sedatives).
Cornea and External Disease Perspective
Steroid penetration through the cornea is quite effective, so cornea specialists often achieve success with topical steroids for ocular surface disease and anterior segment inflammation. “We’re fortunate not to have to deal with systemic side effects or the treatment burden of multiple injections,” said Dr. McLeod.
When choosing a steroidal agent, Dr. McLeod considers a combination of potency and penetration. For high potency and penetration, he uses prednisolone acetate or prednisolone phosphate; if surface activity is especially important, he uses fluorometholone. Dr. Sheppard prefers difluprednate for potency and induction therapy, and loteprednol etabonate for surface activity and maintenance therapy. (See “Anti-Inflammatory Potency of Topical Ophthalmic Steroids.”)
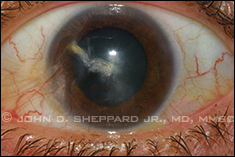 |
Central corneal herpetic stromal scar
with neovascularization. |
THE STEROIDS FOR CORNEAL ULCERS TRIAL (SCUT).7 The benefits of using steroids to treat keratitis include reductions in inflammation, corneal scarring, and neovascularization. The negatives include heightened risk of infections, indolent ulcers, recurrent ulcers, perforations, endophthalmitis, and impaired re-epithelialization.
Dr. McLeod, who was one of the SCUT investigators, explained that the study was designed to determine whether adding topical steroids to the treatment of a bacterial corneal ulcer would improve post-treatment visual acuity. “We thought we would find that steroids would be associated with a higher complication rate in some patients, but that, overall, steroids would reduce the scarring associated with infection and thus lead to better vision.”
The SCUT actually showed that steroids were not associated with higher complication rates. Nor were they associated with any benefit overall. However, a subset of more severe ulcers with central axial involvement did benefit from steroids—and these are the cases clinicians worry about most.
Because the study was not designed to compare the usefulness of steroids in more severe vs. less severe ulcers, Dr. McLeod advises caution when drawing conclusions.
“What I took from the study is that once you’ve identified the causative organism and confirmed that it’s bacterial, you can have a reasonably short period of getting the antibiotics on board, then add steroids quickly. Given that there seems to be a subset of cases where steroids are helpful, I’m now more comfortable with earlier use of steroids, specifically in bacterial keratitis.” The key is confirming that the keratitis is bacterial because the use of steroids with other types of microbial keratitis, such as fungal or Acanthamoeba, is very worrisome, said Dr. McLeod.
WHEN ARE STEROIDS APPROPRIATE? When inflammation in and of itself threatens eye structure and vision, using steroids is important, noted Dr. McLeod. For example, they should be used for bacterial keratitis, in which corneal scarring is a concern. But for diseases that don’t threaten the eye structurally, the risks of steroids can outweigh the benefits.
Conjunctivitis and blepharitis. For bacterial conjunctivitis, a course of antibiotics typically is sufficient; the same is true for bacterial blepharitis, according to Dr. McLeod. “But for blepharitis with a fair amount of skin involvement, the irritation can be quite trying for patients,” he said. “In those cases, steroids can be helpful in shortening disease course and alleviating discomfort.”
Chalazion and vernal keratoconjunctivitis. Steroid therapy is used in nearly all cases of chalazion and vernal keratoconjunctivitis.
Dr. McLeod noted that “the fundamental pathology of a chalazion is the inflammatory reaction, so when it fails to respond to hot compresses and massage, I do intralesional steroid injections. Despite the potential side effect of depigmentation, this can effectively manage tenacious chalazia.”
For vernal keratoconjunctivitis, which can be accompanied by a great deal of inflammatory change (especially in children) on the undersurface of the tarsal conjunctiva, it’s particularly useful to inject steroids directly into the supratarsal space,8 said Dr. McLeod.
Dry eye. Steroid therapy for dry eye is controversial. Many clinicians treat this condition with cyclosporine, either alone or in combination with a steroid. Dr. McLeod does not. “In our practice, we haven’t experienced a robust clinical response to either,” he said.
On the other hand, Dr. Sheppard reports successful outcomes when using cyclosporine in patients with dry eye who have pure aqueous tear deficiency. For patients with dry eye accompanied by redness, blepharitis, significant tarsal changes, or ocular allergy, he administers induction therapy with a topical steroid at one visit and then maintains them on cyclosporine for the long term. Once the patients are in a successful maintenance phase, Dr. Sheppard recommends that they use their steroid for acute flare-ups triggered by travel, allergies, respiratory infection, or exposure to environmental irritants. His steroid of choice for this indication is loteprednol.
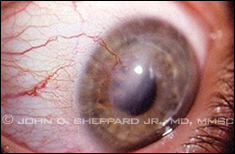 |
Corneal allograft rejection adjacent to
neovascularization. |
Corneal transplantation. The mainstay for graft preservation is topical steroids, even in the event of an acute immunologic attack. “There is no clear evidence in the literature that adding oral or intravenous steroids makes a significant difference,” said Dr. McLeod.
A bigger question is how long to keep the patient on steroids after a transplant. “For young, phakic patients, I try to get them off steroids after a few months because of cataract risk,” said Dr. McLeod. “But in older patients, especially if they’re pseudophakic, I recommend chronic prophylaxis as long as IOP allows it.”
Many comprehensive ophthalmologists are not comfortable managing steroids indefinitely and may discontinue them. This is inadvisable because most cases of rejection occur in patients who stopped using steroids. At the first sign of rejection—reduced vision, graft thickening, or photophobia—steroids should be started immediately, said Dr. McLeod.
Sins of omission and commission. For patients with Thygeson superficial punctate keratitis (TSPK), some ophthalmologists steer clear of steroids because they worry that the lumps will return or become more persistent. However, Dr. McLeod has found that judicious use is very helpful in alleviating discomfort. Topical cyclosporine also has been used effectively for TSPK, according to Dr. Sheppard.
Dr. McLeod sometimes sees a patient with a corneal dendrite that was overlooked, and the patient is being treated with steroids for epithelial keratitis. This is definitely not recommended.
However, steroids do have a role in a specific type of epithelial herpetic keratitis: persistent epithelial disease with underlying anterior stromal inflammation. In that condition, it’s very difficult for the epithelium to close over. “We definitely want to stay away from steroids in pure epithelial herpetic disease,” noted Dr. McLeod, “but if we have more persistent disease with underlying stromal inflammation that seems to be providing an inhospitable environment for re-epithelialization, then a little bit of steroid can be helpful.” Concomitant topical or systemic antivirals must be used whenever steroids are prescribed for herpetic keratitis.
Uveitis Key Points
“Almost all the principles of steroid use have derived from the treatment of uveitis,” said Dr. Sheppard. “It has served as a laboratory for evaluating inflammation.”
With uveitis, you must answer the following three questions:
- Is there a systemic association?
- Is there an infectious component?
- Is the patient at risk for recurrence or chronic inflammatory disease?
The answers can help you choose the appropriate treatment, said Dr. Sheppard. This algorithm also applies to scleritis, noted Dr. McLeod.
If the uveitis has an infectious component (e.g., systemic syphilis, toxoplasmosis, or Lyme disease), you still need to treat the inflammatory process, said Dr. Sheppard. But you must treat concomitantly with aggressive antibiotic therapy.
For uveitic conditions caused by an overactive immune system, such as ankylosing spondylitis, Vogt-Koyanagi-Harada syndrome, juvenile idiopathic arthritis, and birdshot chorioretinopathy, you need steroids for induction therapy and steroid-sparing immunosuppressive agents for maintenance therapy, said Dr. Sheppard. “Our primary directive is to reduce oral steroids to 7.5 mg per day or less, as quickly and as safely as possible, with or without adjunctive oral, implantable, injectable, or topical agents.”
When lecturing about uveitis treatment, Dr. Sheppard emphasizes zero tolerance for inflammation. “Patients have to be treated a bit beyond resolution before you start weaning—and then you wean with a controlled stepwise plan.”
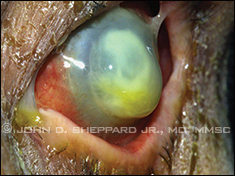 |
Necrotizing Pseudomonas keratitis
prior to perforation. |
INTRAVITREAL IMPLANTS. Sustained-release corticosteroid implants are an exciting development for long-term therapy and are appropriate for advanced noninfectious posterior uveitis. “They’re truly a godsend for a distinct, albeit small, group of the most severe cases,” said Dr. Sheppard.
Implants guarantee compliance, provide continuous dosing, avoid systemic toxicity, bypass gastrointestinal absorption, and eliminate the risks associated with topical toxicity, Dr. Sheppard said. Although sustained delivery also dramatically decreases treatment burden, the drawback is that you can’t titrate the dose, said Dr. Morse.
A fluocinolone acetonide implant (Retisert) and a dexamethasone implant (Ozurdex) are currently approved in the United States. Retisert, which is implanted surgically, has a much longer duration of action (up to three years) than the dexamethasone implant (about four to six months), which is administered by injection. However, dexamethasone is more potent.1 Another fluocinolone implant, Iluvien, has a duration equal to that of Retisert and can be injected in the clinic through a 25-gauge needle, avoiding a trip to the operating room. It is currently used in Europe but not yet approved in the United States.
(For a detailed discussion of treatments for noninfectious uveitis, see EyeNet’s October 2012 feature article.)
Vitreoretinal Hot Topics
Intravitreal steroids are major players in retinal practice, beyond posterior uveitis. They are used to treat cystoid macular edema secondary to diabetes, retinal vein occlusions, exudative macular degeneration, and pseudophakia.1
After the advent of anti-VEGF therapies, steroids had a reduced role in retinal practice, said Dr. Morse, mainly because of their side effects. “But steroids are having a resurrection because there’s now widespread recognition that inflammation is present in diabetes, vein occlusions, macular degeneration, and even retinal dystrophies,” he said. “Some patients don’t respond to anti-VEGFs, indicating that inflammation may be the primary pathogenic mechanism in what’s causing their ocular problems.”
SUSTAINED VS. NONSUSTAINED DELIVERY. Because topical steroids don’t usually reach therapeutic levels in the posterior segment, intraocular administration is the preferred route for retinal disease, said Dr. Morse.
The steroids used most frequently for intraocular administration are triamcinolone and dexamethasone, which may be given as injections (Triesence and Kenalog, respectively). Triamcinolone is believed to have high activity for two months; dexamethasone has a shorter clinical effect. Retisert (fluocinolone) and Ozurdex (dexamethasone), the sustained-release implants discussed in the uveitis section, are used for vitreoretinal disorders, as well.
How do you determine whether to use sustained or nonsustained delivery? “I like to start with a short-term agent—one that lasts a couple of months—just to see if the agent is going to work. I use 1 mg of Triesence for intravitreal injections and 40 mg of Kenalog for posterior sub-Tenon steroid injections. It’s far more economical than using a sustained-delivery system right away and gives me an idea of what sort of response the patient might have to the steroid,” said Dr. Morse.
“Whether to then use sustained or nonsustained delivery depends on the condition being treated. For example, if a case has failed multiple lasers and multiple anti-VEGF therapies, we’ll probably need to treat it for a longer time, and sustained delivery makes sense,” he said. Indications for sustained delivery include retinal vein occlusion, diabetic macular edema, and chronic inflammatory conditions such as uveitis.
Dr. Morse has seen dramatic improvements with the dexamethasone implant when treating such conditions. He added a caution, however: “An implant is highly desirable, but once it is in there, you don’t want to go fishing it out if there are untoward side effects.”
COMBINATION THERAPY. “Unless there’s a clear indication that you’re dealing with a marked uveitic or inflammatory component to a disease, steroids may not be the best first-line therapy for retinal conditions,” said Dr. Morse. But they often have a complementary role. “With macular edema, for example, anti-VEGF agents work well when it’s caused by VEGF. But when you consider your treatment goals, you might also want to use steroids for their anti-inflammatory and neuroprotective benefits,” he said.
Dr. Morse prefers to start with monotherapy, but sometimes it is not sufficient. For treating macular edema from diabetes or vein occlusion, he frequently combines intravitreal triamcinolone (1 mg) with anti-VEGF agents and then may use a focal laser later on. Using the steroid and anti-VEGF agent first can often reduce the macular edema, enabling a lower-power-density laser treatment that results in less retinal damage.
The new application of sustained-delivery steroids for diabetic macular edema is promising and may be useful for reducing treatment burden in the near future, said Dr. Morse.
Neuro-Ophthalmology Viewpoint
Neuro-ophthalmology used to (and sometimes still does) have the reputation of “Diagnose and adios!” But steroids have helped to refute that perception, said Dr. Friedman.
Steroids are the mainstay of treatment for giant cell arteritis (GCA), inflammatory orbital pseudotumor, Tolosa-Hunt syndrome (THS), optic neuritis, trochleitis, and ophthalmoplegic migraine. The steroids convey fast and dramatic results for many patients who have these conditions. “It’s like magic,” said Dr. Friedman.
“Most of the time, steroids are administered orally or intravenously, so we’re talking about all the risks associated with systemic steroids,” said Dr. Friedman. Not only are the dosages relatively high, but most patients with GCA are elderly, which increases the risks even further. That said, the steroids are critical for preventing blindness.
When treating any of these conditions, Dr. Friedman recommends working with an internist to manage the complications that can arise. “The internist can help monitor the patient’s blood pressure, blood glucose, bone density, et cetera. Patients should be put on proton-pump inhibitors to protect their stomachs and on calcium and vitamin D supplements (and maybe bisphosphonates) to protect their bones.”
TREATMENT PARADIGM. Treatment for neuro-ophthalmic inflammatory conditions follows the “hit hard, hit fast, taper slowly” rule.
GCA. “Say a patient presents with vision loss in one eye, and you’re highly suspicious of GCA. Even before the diagnosis is confirmed, you should start the steroid treatment because it takes a few days to get the biopsy results,” said Dr. Friedman. “You don’t want the patient to go blind in the other eye while you’re waiting for the results.”
Dr. Friedman also mentioned that you can start with either intravenous methylprednisolone (usually 1 g, either all at once or in divided doses) or oral prednisone (usually about 80-100 mg per day, with the first dose taken immediately). She added, “Patients stay on a pretty high dose of prednisone until we taper, and everyone has their own personal recipe for tapering.”
Generally, Dr. Friedman keeps her patients on the starting dose of prednisone for at least a few weeks, then starts to taper over a few months (by 10-20 mg every few weeks) to get the maintenance dosage down to between 10 and 20 mg per day. In patients with an abnormal erythrocyte sedimentation rate (ESR), she’ll follow the sedimentation rate as she tapers to ensure that she isn’t moving too quickly. But not everyone has an abnormal ESR, in which case she carefully monitors clinical signs.
Patients with GCA need to be on steroids for 1 to 1.5 years; after that, the last 10 mg are tapered very slowly. Dr. Friedman usually tapers by 1 mg per week at that point. “Ten milligrams is about what your body makes physiologically, but you have to tell your adrenal glands to start working again,” she explained.
To minimize the risks associated with prolonged use of steroids, sometimes steroid-sparing medications are used, such as methotrexate. But the research on methotrexate as monotherapy has yielded disappointing results. Therefore, said Dr. Friedman, patients typically need to stay on prednisone along with the methotrexate, but a lower, safer steroid dose can be used.
Optic neuritis. Unlike GCA, the problem in optic neuritis isn’t with blood supply to the nerve but rather with inflammation and demyelination; therefore, the prognosis is much better than with GCA, said Dr. Friedman. Patients with optic neuritis usually have pain in or around the eye, often with eye movement. Vision loss is generally only in one eye at a time, progressing over hours to days.
Dr. Friedman’s approach is based on the Optic Neuritis Treatment Trial: 1 g per day of methylprednisolone for three days, followed by 1 mg/kg per day of prednisone tapered over 10 days, at which point it is discontinued.9
Orbital pseudotumor and THS. Treatment for orbital pseudotumor and THS (essentially the same condition in different locations) starts the same way as induction therapy for GCA: 80 to 100 mg of prednisone per day. But it is tapered much more quickly than in GCA. “Usually you can get the patients off steroids within a few months, but the bad news is that it tends to come back as you’re tapering the steroids. In that case, we may increase the steroid dose again and start steroid-sparing agents,” said Dr. Friedman, who noted that both conditions are diagnoses of exclusion. “Sometimes lymphoma can masquerade as one of these, so if a patient relapses after coming off steroids, think about lymphoma.”
___________________________
1 Abelson MB, Butrus S. Corticosteroids in ophthalmic practice. Chapter 23. In: Albert DM et al., eds. Albert & Jakobiec’s Principles and Practice of Ophthalmology, 3rd ed. Philadelphia: Saunders Elsevier; 2008.
2 Flach AJ. Cutan Ocul Toxicol. 1991;10(4):253-277.
3 Duvall B, Kershner R. Ophthalmic Medications and Pharmacology, 2nd ed. Thorofare, N.J.: Slack; 2006.
4 Friedman NJ, Kaiser PK. Ocular pharmacology. In: Essentials of Ophthalmology. Philadelphia: Elsevier; 2007:25-32.
5 El-Harazi SM, Feldman RM. Curr Opin Ophthalmol. 2001;12(1):4-8.
6 Simone JN, Whitacre MM. Curr Opin Ophthalmol. 2001;12(1):63-67.
7 Srinivasan M et al. Arch Ophthalmol. 2012;130(2):151-157.
8 Holsclaw DS et al. Am J Ophthalmol. 1996;121(3):243-249.
9 Beck RW et al. N Engl J Med. 1992;326(9):581-588.
Meet the Experts
DEBORAH I. FRIEDMAN, MD, MPH Professor of neurology and neurotherapeutics and ophthalmology, University of Texas Southwestern Medical School, Dallas. Financial disclosure: None.
STEPHEN D. MCLEOD, MD Professor and chairman of ophthalmology, University of California, San Francisco. Financial disclosure: None.
LAWRENCE S. MORSE, MD, PHD Professor of ophthalmology and vitreoretinal fellowship director of retina services, University of California Davis School of Medicine, Sacramento. Financial disclosure: Received grant support from and serves on the medical advisory board of Allergan.
JOHN D. SHEPPARD JR., MD, MMSC Professor of ophthalmology, microbiology, and immunology and clinical director of the Lee Center for Ocular Pharmacology at the Eastern Virginia School of Medicine, Norfolk. Financial disclosure: Is a consultant for or has clinical research affiliations with Abbott, Alcon, Allergan, Aseoptics, Bausch + Lomb, Eleven Biotherapeutics, EyeGate, EyeRx Research, Lux Bio, Merck, OcuCure, Otsuka, Santen, SARcode, ScienceBased Health, TearLab, and Vistakon.
|