By Renu V. Jivrajka, MD, and Ahmad A. Aref, MD
Edited by Sharon Fekrat, MD, and Ingrid U. Scott, MD, MPH
Download PDF
Open-angle glaucomas are typically classified as either primary or secondary. Primary open-angle glaucoma is a diagnosis of exclusion, characterized by an open anterior chamber angle and lack of a secondary, contributing disease process. Secondary glaucomas are related to an underlying disease that causes increased resistance to trabecular meshwork outflow and subsequent intraocular pressure rise. Examples of secondary glaucomas include the pigmentary, phacolytic, steroid-induced, exfoliation, neovascular, and angle-recession types.
An important cause of pigmentary glaucoma relates to pigment release from the posterior iris surface due to direct contact with an improperly positioned IOL in the ciliary sulcus. This article highlights the nature of this process and includes recommendations for prevention and management.
Pathophysiology
A compromised posterior capsule at the time of phacoemulsification often precludes placement of an IOL in the capsular bag and may require placement of a sulcus IOL for adequate visual rehabilitation. Several IOLs are available to be placed in the ciliary sulcus, one of which is the single-piece acrylic IOL. This is a poor choice, however, due to its square-edged optic design, thick haptics, and unpolished side walls. These design characteristics lead to friction at the edges of the lens and subsequent pigment epithelial disruption of the iris and ciliary body.1 The disrupted pigment is then deposited on anterior chamber structures and can lead to obstruction of aqueous outflow, IOP rise, and a glaucomatous optic neuropathy. Moreover, the highly flexible yet bulky haptics of the single-piece acrylic IOL are prone to decentration when placed in the sulcus, which can exacerbate the release of pigment through increasing contact with the posterior iris.
Differential Diagnosis
Pigment dispersion syndrome. Individuals with pigment dispersion syndrome (PDS) develop pigment deposition on many structures. Clinically, these patients may present with a Krukenberg spindle as well as midperipheral iris transillumination defects. (See also “Morning Rounds.")
Unlike patients with secondary glaucoma from single-piece sulcus IOLs, patients with PDS demonstrate iris transillumination defects in a spokelike pattern. On gonioscopy, they may have a Sampaolesi line in addition to a diffusely pigmented trabecular meshwork. Furthermore, with dilation, patients can develop pigment deposits over both the zonular fibers and the anterior hyaloid face.
Uveitis-glaucoma-hyphema. This condition, known as UGH, is another form of secondary glaucoma due to chronic irritation from a malpositioned or rotated anterior chamber IOL. Clinical features include chronic inflammation, secondary iris neovascularization, and recurrent hyphemas. UGH may require surgical intervention to reposition or exchange the lens, which can be technically challenging when the IOL is in the sulcus. Furthermore, the presence of synechiae or an open posterior capsule makes for a less-than-ideal surgical circumstance.
Surgical trauma. Operative procedures can result in a postoperative IOP rise. Often, the exact mechanism is unknown, but these episodes tend to be transient. Pigment release, inflammatory cellular reaction, debris deposition, and mechanical deformation of the trabecular meshwork can all contribute to the IOP rise.
Typically, IOP spikes occur within the immediate postoperative period. In contrast, cases of secondary glaucoma following placement of a single-piece IOL in the sulcus can occur months to years later, with an intermittent release of pigment causing a wide range of IOP fluctuation.
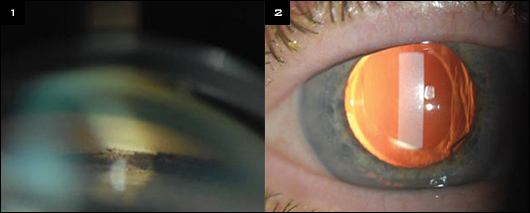 |
|
FINDINGS. (1) Gonioscopy of the anterior chamber angle found pigment deposition in the trabecular meshwork secondary to an improperly positioned single-piece acrylic IOL in the ciliary sulcus space. (2) Slit-lamp photo of a characteristic iris transillumination defect in the shape of an IOL haptic in the ciliary sulcus.
|
Clinical Findings
In order to establish a diagnosis of secondary glaucoma related to a single-piece sulcus IOL, it is important to exclude a diagnosis of PDS. Patients should also have a normal preoperative exam as well as no evidence of secondary glaucoma in the fellow eye after surgery.
In addition, other causes of secondary pigment dispersion, such as intraoperative iris trauma or iris prolapse, should be excluded.
Patients with a single-piece IOL in the ciliary sulcus may present with a Krukenberg spindle and hyperpigmentation of the trabecular meshwork on clinical exam (Fig. 1). On gonioscopy, this pigmentation may be highly variegated, with excess pigment localized to the segments adjacent to where the IOL haptics are lodged in the sulcus.
A common exam finding using slit-lamp biomicroscopy is a characteristic iris transillumination defect. This feature is best seen through an undilated pupil using a slit beam perpendicular to the iris plane. As a result of iris chafing by the IOL haptic in the sulcus, the iris transillumination defect can be wedge shaped (Fig. 2).
Other complications from sulcus placement of single-piece IOLs may include recurrent iridocyclitis, cystoid macular edema, and, most commonly, lens decentration resulting in symptomatic edge glare. The contact of the sharp IOL edges with the posterior iris vasculature can also predispose the eye to chronic uveal inflammation and recurrent microhyphemas that can impair vision and abruptly raise the IOP.2
Management
The majority of cases of secondary glaucoma arising from single-piece IOLs placed in the sulcus require explantation of the IOL. In histopathologic analyses of these lenses, pigment granules have been found on the anterior surface of the IOL, with the bulk of the granules found at the haptic-optic junction. The histology findings correlate with the proposed mechanism of posterior iris chafing as the leading cause of pigment release and secondary glaucoma.3
Even in eyes with secure sulcus fixation, frequent monitoring for signs of chronic inflammation and pigment dispersion is vital in order to avoid permanent vision loss from elevated IOP. When necessary, an IOL exchange using a three-piece IOL with a smooth anterior optic surface and rounded edges should be performed to minimize any iris chafing.
IOL Design Notes
Three key design features determine the biomechanical behavior of an IOL: 1) The haptics are intended to exert pressure on the eye structures for IOL fixation. With haptic compression, a certain amount of stress relaxation occurs to aid in fixation and positional stability. 2) Foldable IOLs are amenable to compression into a smaller overall diameter by the tissues in the eye. 3) The haptics are constructed to withstand manipulation during handling and exposure to different temperatures.
The ultimate clinical goal is to obtain a stable and consistent IOL position within the lens capsule in order to minimize any undesirable postoperative refractive error. The single-piece acrylic IOLs are widely used because of their biomechanical advantages over other IOLs—in particular, their planar haptics limit axial displacement and have superior durability.1 However, these advantages are only realized when the single-piece acrylic IOL is properly positioned in the capsular bag.
___________________________
1 Lane S et al. J Cataract Refract Surg. 2004;30(11):2397-2402.
|
Summary
The previous belief that a single-piece acrylic IOL is suitable for sulcus fixation has been shown to be untrue. We now know that implanting this device may lead to iris pigment release and secondary glaucoma, which can develop years later. Alternative approaches should be considered in situations in which IOL placement in the capsular bag is not feasible.
___________________________
1 LeBoyer RM et al. J Cataract Refract Surg. 2005;31(7):1421-1427.
2 Chang D et al. J Cataract Refract Surg. 2009;35(8):1445-1458.
3 Hadid O et al. J Cataract Refract Surg. 2010;36(9):1610-1611.
___________________________
Dr. Jivrajka is a third-year ophthalmology resident and Dr. Aref is assistant professor of ophthalmology; both are at the University of Illinois Eye and Ear Infirmary in Chicago. The authors report no related financial interests.
Got Pearls?
INTERESTED IN SHARING TIPS WITH YOUR COLLEAGUES?
Write a Pearls article!
Ophthalmic Pearls
EyeNet Magazine
655 Beach Street
San Francisco, CA 94109
eyenet@aao.org
Writers guidelines provided upon request.
ARE YOU A RESIDENT?
An article published in Ophthalmic Pearls will satisfy the RRC requirements for resident scholarly activity.
|