Download PDF
A 39 years old, Jane Culpepper* had a hectic schedule. Indeed, the up-and-coming chef never felt like she had the time to make her medical appointments while working to provide for her family. But after four days of acute, progressive diplopia, she had reached her limit.
Our First Encounter
When Ms. Culpepper presented at the emergency department (ED), she said that she had had double vision for four days. This had been constant in all fields of gaze with no change in symptoms throughout the day. She also mentioned that in the previous two to three days, her right eyelid had become somewhat tender and droopy. She noted that within the past month she had symptoms of an upper respiratory infection and had received the first dose of a COVID-19 vaccination.
Initial exam. Notably, Ms. Culpepper had a past diagnosis of hypertension, and her blood pressure at presentation in the ED was 182/108 mm Hg. The ophthalmology resident on call noted that Ms. Culpepper’s visual acuity was 20/20 in both eyes and her base exam was normal apart from eye movements: Testing of her extraocular movements revealed a mild restriction, graded at –1, in all abduction movements in the right eye. The resident also noted that Ms. Culpepper’s diplopia did not resolve with pinhole.
The remainder of her systemic neurological exam was within normal limits.
Initial diagnosis. Ms. Culpepper was diagnosed with a right sixth nerve palsy, which—given her elevated blood pressure—was thought to be secondary to hypertensive ischemia. The remainder of her systemic neurological exam, which was performed by a neurology resident, was within normal limits.
Laboratory tests. A battery of screening labs was ordered, revealing an elevated hemoglobin A1c and elevated erythrocyte sedimentation rate (ESR) to 78 mm/hour. C-reactive protein (CRP) was mildly elevated at 1.3 mg/mL, and her vitamin D level and thyroid-stimulating hormone (TSH) were within normal limits. An antithyroglobulin antibody test was nonreactive.
Imaging. Magnetic resonance imaging (MRI) of Ms. Culpepper’s brain and orbits showed nonspecific findings that possibly represented demyelinating disease, microvascular ischemia, or vasculitis. Ms. Culpepper was started on hypertensive medication and discharged. Before she left, she was scheduled her for a clinic appointment with our ophthalmology department, and she also was assigned a new primary care provider for control of her blood pressure.
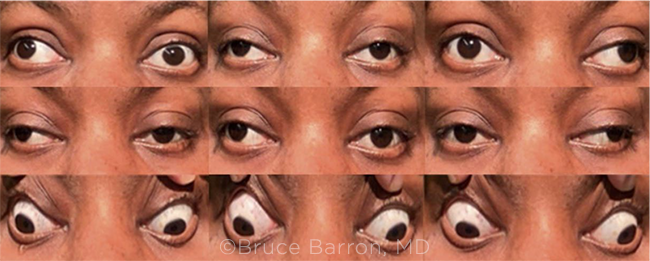 |
|
WE GET A CLOSER LOOK. Two weeks after the patient’s initial presentation to the ED, we noted right exotropia in primary gaze. We also saw bilateral adduction deficits, along with bilateral upgaze limitation.
|
We Get a Closer Look
Two weeks later, Ms. Culpepper came to the ophthalmology clinic for follow-up. She had experienced a significant decline in function since the ED visit and now reported daily headaches, paresthesias, unprovoked falls, and a worsening of her diplopia.
Results of our anterior segment exam and direct fundus exam were consistent with those from her ED visit. However, extraocular muscle function testing showed notable changes (Fig. 1). She had bilateral adduction deficits, limitation in upgaze bilaterally, and an alternating exotropia.
A full neurological exam in clinic revealed the following: Cranial nerves II, V, and VII-XII were found to be intact bilaterally. However, when examining cranial nerves III, IV, and VI, she was found to have bilateral adduction deficits and limitation in upward gaze.
Her motor exam was grossly intact without atrophy, fasciculations, or rigidity. Her strength was graded 5/5 throughout upper and lower extremities. Her reflexes revealed 1+ biceps, brachioradialis, and triceps reflex. She was areflexic bilaterally in the patellar and Achilles’ tendons.
Her sensory exam showed light touch, pain, and temperature to be intact. She had decreased vibratory sense bilaterally in her upper and lower extremities. A cerebellar exam was within normal limits.
After we had a thorough discussion with the staff neuro-ophthalmologist, Ms. Culpepper was emergently sent to the ED for further workup and admission.
On admission, she received a repeat MRI series of the brain, orbits, and spine; these showed no acute processes. Additional lab testing included antithyroperoxidase (TPO) antibody, angiotensin-converting enzyme (ACE) level, a myasthenia gravis panel, a demyelinating disease panel, and a panel for anti-GQ1b syndromes.
Differential Diagnosis
While the differential for Ms. Culpepper’s presentation was large, our team pursued a workup for causes of a “pseudo” internuclear ophthalmoplegia, as Ms. Culpepper had no findings of ischemia or demyelination of the medial longitudinal fasciculi (MLFs).
Myasthenia gravis. Myasthenia gravis is an autoimmune disorder that primarily affects the neuromuscular junctions (NMJs) of muscle fibers. Auto-antibodies against postsynaptic acetylcholine receptors at these NMJs are formed, which is the basis of this disease. Extraocular muscles (EOMs) are often affected, as their twitch fibers develop tension faster and have a higher frequency of synaptic firing than other appendicular and axial muscles. Because of this, EOMs are more susceptible to fatigue.1
Miller Fisher syndrome. Miller Fisher syndrome (MFS) is an autoimmune, antibody-mediated neurologic disorder. It is part of a group of diseases known as the anti-GQ1b syndromes. The syndrome’s typical presentation is a triad of symptoms including ophthalmoplegia, ataxia, and areflexia. Through the mechanism of “molecular mimicry,” GQ1b antibodies develop in response to the infectious agents. They cross-react with the gangliosides on cranial nerves III, IV, and VI, muscle spindle afferent fibers, cerebellar granule cells, and dorsal root ganglia. This disease is typically self-resolving.
Cavernous sinus syndrome. Cavernous sinus syndrome is a condition caused by any pathology involving the cavernous sinus. It can present as unilateral ophthalmoplegia of the third, fourth, or sixth cranial nerves, autonomic dysfunction, or sensory trigeminal (V1-V2) loss. Because of the wide variety of structures that pass through the cavernous sinus, the presentation of this disease can vary greatly.
Nonspecific orbital inflammation. Nonspecific orbital inflammation (NSOI) is a benign, noninfectious process of the orbit. It is typically a diagnosis of exclusion, and its etiology is unknown. The disease consists of a lymphocytic inflammatory response with varying degrees of fibrosis. The inflammation caused by NSOI can affect any of the EOMs, also causing a wide range of presentation.2
Making the Diagnosis
While waiting for Ms. Culpepper’s lab work to come back, we started her on pyridostigmine and prednisone for presumed myasthenia gravis. However,this treatment didn’t improve her condition, and she experienced severe nausea. Her workup for myasthenia gravis eventually came back negative.
Electromyography (EMG) revealed sensory axonal neuropathy, left carpal tunnel syndrome affecting sensory nerves, absence of features of demyelination, and absent F waves.
The physical exam and EMG findings, plus an anti-GQ1b antibody panel positive for IgM and IgG, added up to the constellation of findings seen in MFS. Elevated ESR has also been reported in MFS patients, which is consistent with the patient’s previously elevated levels.
After making the diagnosis of MFS, we started Ms. Culpepper on a series of four 0.5 mg/kg intravenous immunoglobin (IV IG) infusions, which resulted in improvement of her ophthalmoplegia.
Discussion
MFS was first described in 1956 by Charles Miller Fisher, MD, as a disease unique from Guillain-Barré syndrome (GBS), with which the triad of ophthalmoplegia, ataxia, and areflexia had already been associated.
Incidence. The incidence of MFS is 1 to 2 cases per 1,000,000, with a reported male predominance.
Pathogenesis. Campylobacter jejuni, cytomegalovirus, and Mycoplasma are frequently suspected organisms in the pathogenesis of MFS and GBS.
Symptoms. Cases are preceded by a respiratory or gastrointestinal illness approximately one or two weeks prior to neurological symptoms. MFS symptoms begin with diplopia and subsequently involve ataxia and areflexia as well as other neurological phenomena, such as mydriasis and sensory losses.3
MFS versus myasthenia gravis. MFS can be confused clinically with myasthenia gravis, as in our case, because of the overlap of ocular findings. Myasthenia gravis can present as isolated ocular findings in 13% of cases and can present as ocular findings accompanied by other neurological deficits in 37% of cases. The findings of ophthalmoplegia, ptosis, and hypotonia in myasthenia gravis may be easily conflated with the ophthalmoplegia and hypotonia seen in MFS. Thus, it is critical to differentiate the two disorders early to avoid misdiagnosis and potentially harmful sequela.
An anti-GQ1b syndrome. The presence of anti-GQ1b antibodies has become the gold standard for diagnosis of MFS. These antibodies are unique to the underlying autoimmune process of MFS. As seen in our case, these antibodies were vital in tying together the constellation of symptoms and determining the correct treatment for our patient.
Treatment. The mainstay of treatment for MFS and GBS involves early infusions of IV IG or exchange plasmapheresis. These options have been shown to be equal in terms of patient outcomes.4
Conclusion
After the series of IV IG infusions, Ms. Culpepper’s neurological symptoms began to wane, and she was discharged from the hospital with appropriate outpatient follow-up. No initial inciting infection could be identified to explain the onset of MFS, and Ms. Culpepper could not recall having any gastrointestinal or respiratory symptoms. However, she had received the Pfizer vaccine for COVID-19 two weeks prior to the initial presentation. MFS has been presented as a rare complication of COVID-19 infection.5 In this case, it would be impossible to determine if the immunologic cascade began as a result of possible COVID-19 infection or COVID-19 vaccination.
___________________________
*Patient name is fictitious.
___________________________
1 Nijsse B et al. BMJ Case Rep. 2014:doi:10.1136/bcr-2013-203234.
2 Tamhanker MA. Eye movement disorders: Third, fourth, and sixth nerve palsies and other causes of diplopia and ocular misalignment. In: Liu GT et al., eds. Liu, Volpe, and Galetta’s Neuro-Ophthalmology. Elsevier; 2019:489-547.
3 Mori M et al. Neurology. 2001;56(8):1104-1106.
4 Al Othman B et al. Curr Opin Ophthalmol. 2019;30(6):462-466. (Published correction appears in Curr Opin Ophthalmol. 2020;31(1):80.)
5 Li Z et al. Environ Sci Pollut Res Int. 2021;28(17):20939-20944.
___________________________
The authors thank Wayne J. Wortmann for his significant contribution to this article. Mr. Wortmann is a fourth-year medical student, Dr. Friend is an ophthalmology resident, and Dr. Al-Dujaili is a glaucoma specialist; all are at Louisiana State University, New Orleans. Financial disclosures: None.