Download PDF
Some evidence suggests that patients with diabetes are at increased risk of developing diabetic retinopathy (DR) following cataract surgery. A recent report confirms this link in Asian patients.1 Even after adjusting for variables, the relative risk of developing DR was higher in eyes that had undergone cataract surgery than in eyes that remained phakic. This finding was observed mainly in cases of mild or moderate DR.
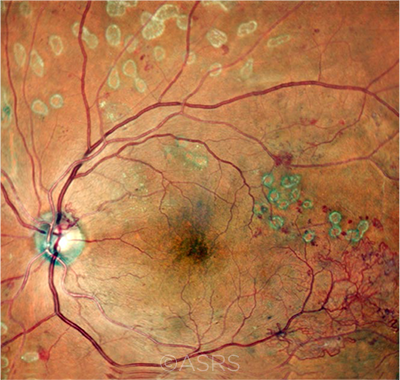 |
PDR. Risk of DR development following cataract surgery was higher in patients with mild or moderate DR, in contrast to the proliferative DR shown here. This image was originally published in the ASRS Retina Image Bank. Gayathri Mohan and Manish Nagpal. Proliferative Diabetic Retinopathy. Retina Image Bank. 2020; Image Number 54853. © The American Society of Retina Specialists.
|
Mining the data. For this population-based study, the researchers recruited 972 Malay and Indian participants (1,734 eyes) with type 2 diabetes from the Singapore Epidemiology of Eye Diseases Study. A total of 350 eyes had undergone cataract surgery, either before baseline or during six years of follow-up. Of those who had undergone cataract surgery, 22% developed DR, compared to 14.1% of eyes that remained phakic through follow-up.
Adjusted covariates significantly associated with increased risk of developing DR included being slightly younger (mean age, 59 vs. 57.7 years old), having a higher hemoglobin A1c level (8.7 vs. 7.4), and having a longer history of diabetes at baseline (6.6 vs. 5.2 years).
Need for additional study. No significant association emerged between cataract surgery and progression of DR, possibly due to the limited statistical power of the data. A meta-analysis or consortium collaboration might address this question, said coauthor Ching-Yu Cheng, MD, PhD, at the Singapore Eye Research Institute.
Dr. Cheng and his colleagues are conducting an additional analysis of the data with 12-year follow-up; this will include a Chinese cohort. They also plan to study the impact of other factors on DR development or progression.
Need to follow diabetic patients. It is too early to generalize the study’s findings to other populations or to issue new clinical guidelines, said Dr. Cheng. In the meantime, he advised that clinicians inform patients with diabetes about the postsurgical risk of developing DR. He also suggested that clinicians should consider careful, and perhaps more frequent, monitoring of diabetic patients following cataract surgery.
—Miriam Karmel
___________________________
1 Tham YC et al. JAMA Network Open. 2020;3(6):e208035.
___________________________
Relevant financial disclosures—Dr. Cheng: None.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
David F. Chang, MD Carl Zeiss: C; Eyenovia: O; iDrops: C,O; Ivantis: C,O; Johnson & Johnson Vision: C; Mynosys: C,O; Perfect Vision: C; PowerVision: C,O; Presbyopia Therapies: O; RxSight: C; Slack: P; Surface: O; Versant Ventures: O; Viewpoint: C,O.
Dr. Cheng None.
Dr. Huxlin Clerio Vision: O. Dr. Huxlin also is coinventor on U.S. Patent No. 7,549,743.
Dr. Medeiros Aerie Pharmaceuticals: C; Allergan: C,S; Annexon: C; Biogen: C; Carl Zeiss: C,S; Galimedix: C; Google: S; Heidelberg: S; IDx: C; nGoggle: P; Novartis: S; Stealth Biotherapeutics: C; Reichert: C,S.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|
More from this month’s News in Review