Download PDF
A retrospective study of diabetic macular edema (DME) patients in a large health care system found that—when compared to patients in landmark clinical trials—these “real-world” patients received fewer intravitreal injections over the first 12 months of treatment, were monitored less frequently, and achieved inferior vision outcomes.1
Too few injections? The findings are comparable to earlier studies based on large Medicare and commercial datasets that reported significant undertreatment of DME with anti–vascular endothelial growth factor (VEGF) drugs.
“There may be widespread underutilization of anti-VEGF agents in treating DME,” said lead author Nancy M. Holekamp, MD, a retina specialist in Chesterfield, Missouri. “I think many of us are not aware of this phenomenon, which may compromise clinical outcomes.”
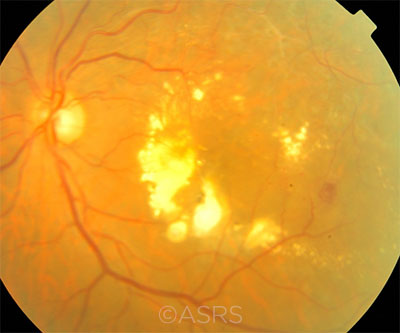 |
DME. Abundant foveal hard exudates in the left eye of a 55-year-old patient with diabetes, hypertension, and normal serum lipid levels. This image was originally published in the ASRS Retina Image Bank. Mallika Goyal, MD. Diabetic Macular Edema. Retina Image Bank. 2012; Image Number 1836. © The American Society of Retina Specialists.
|
Study details. The study involved 110 patients (121 eyes) who received intravitreal anti-VEGF therapy for DME with either bevacizumab or ranibizumab from January 2007 through May 2012 (aflibercept was not available during this time). Most eyes (n = 116, 95.9%) received bevacizumab.
Surprise outcome. “The most surprising finding was the extremely low number of anti-VEGF injections given to DME patients in the first year of treatment,” Dr. Holekamp said. For instance, the mean number of injections was 3.1 (range, 1-12, versus 9-12 in landmark trials such as RISE/RIDE).
Additional findings. Other outcomes of note include the following:
- More than 68% of the eyes received 3 or fewer injections, and just 3% received 10 or more injections.
- Visual acuity improved by 4.7 Early Treatment Diabetic Retinopathy Study letters (converted from Snellen charts to approximate ETDRS letter scores), compared to an average of about 12.0 ETDRS letters in RISE/RIDE.
- The percentage of eyes losing ≥10 or ≥15 letters was 10.8% and 8.3%, respectively, about 2-fold higher than clinical trial eyes.
- Only 59% of patients had regular (at least quarterly) visits, while fewer than 2% had monthly visits, comparable to patients in the landmark trials.
Clinical implications. Dr. Holekamp said she hopes the study raises awareness “of our potential deficits as practicing retina specialists.” She advised her colleagues to pay attention to their DME treatment patterns: “Look back over a year of treating each individual patient. Did you see the patient often enough? Did you give a sufficient number of injections to give this patient the very best chance of gaining and maintaining vision?”
—Miriam Karmel
___________________________
1 Holekamp NM et al. Am J Ophthalmol. Published online April 20, 2018.
___________________________
Relevant financial disclosures—Dr. Holekamp: Alimera Sciences: C,L,S; Allergan: C,L,S; BioTime: C; Genentech: C,L,S; Katalyst: C,P; NotalVision: S; Novartis: C; Ophthotech: S; Ohr Pharmaceuticals: S; Regeneron: C,L.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Chang Carl Zeiss: C; Eyenovia: O; Iantech: C,O; Icon Bioscience: O; iDrops: C,O; Ivantis: C,O; Johnson & Johnson Vision: C; Mynosys: O,C; PowerVision: C,O; Presbyopia Therapies: O; RxSight: O,C; Slack: P; Versant Ventures: O.
Dr. Holekamp Alimera Sciences: C,L,S; Allergan: C,L,S; BioTime: C; Genentech: C,L,S; Katalyst: C,P; NotalVision: S; Novartis: C; Ophthotech: S; Ohr Pharmaceutical: S; Regeneron: C,L.
Dr. Shantha Santen: C.
Dr. Yeh Alcon: S; Clearside Biomedical: C; Santen: C.
Dr. Yin None.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|
More from this month’s News in Review