Download PDF
Special Considerations in Cataract Surgery is an occasional series on challenging situations in cataract surgery. Future installments will cover such topics as cataract surgery in patients with uveitis and other conditions.
Coexisting retinal disease makes cataract surgery a greater challenge than it would normally be for the surgeon and a riskier prospect for the patient. But ophthalmologists who perform cataract surgery can minimize the impact of this extra disease burden on visual outcomes by modifying their pre-, peri-, and postoperative routines, experts in complex ocular surgery say.
A Note on Informed Consent
When a patient facing cataract surgery has a coexisting retinal disease, the surgeon’s informed consent procedures must go beyond the basics, said Adrienne Williams Scott, MD, at the Wilmer Eye Institute. In particular, consent can emerge as a contentious issue in cases of age-related macular degeneration (see “Eyes With AMD”).
“We need to manage expectations. These are patients with whom I have a long discussion about the ultimate visual outcome. If patients have something like a macular hole or have had previous retinal surgery, they might not have the same potential” with regard to visual outcome as other patients do, she said, and this needs to be made clear.
“You cannot overemphasize the importance of the informed consent with these complex patients,” agreed Thomas A. Oetting, MD, at the University of Iowa. "If the preoperative informed consent was adequate, then your patient will better accept that a particular postoperative issue is something you expected. They’ll know that the cataract surgery itself isn’t to blame—that you’re not just bringing up an excuse.”
Eyes With Prior Retinal Surgery
In addition to affecting the duration and outcome of the patient’s visual recovery from cataract surgery, a previous retinal surgery can alter the approach to the surgery itself, Dr. Scott said. “It’s important for the anterior segment surgeon to understand what kind of procedure the patient had and consider this in the surgical plan.”
After scleral buckle. Because a scleral buckle makes the axial length longer, it affects IOL power calculations, Dr. Scott noted. A buckle procedure also can cause conjunctival scarring, and it increases the risk of scleral perforation with injection anesthesia.1
After vitrectomy. These eyes require caution and gentle intraoperative technique because the globe is softer than normal and has unstable zonules and a deeper anterior chamber—and these factors raise the possibility of intraoperative miosis and fluctuations in chamber depth, Dr. Scott said. “We try very hard to avoid contacting the posterior capsule during surgery, but posterior capsule instability is something that the surgeon should watch out for in vitrectomized eyes.”
Dr. Oetting noted that extra caution is required when a cataract develops soon after a vitrectomy is performed, because a rapid cataract can be caused by injury to the posterior capsule during vitrectomy. Surgeons should be particularly careful with hydrodissection and should treat these cases much like a posterior polar cataract, he said.
In addition, the most common issue with postvitrectomy cataract surgery is the mobility of the posterior capsule, which can lead to capsular tear if the surgeon is not careful, Dr. Oetting said. He often will place a capsular tension ring in these eyes.
|
Supportive Strategy
|
 |
|
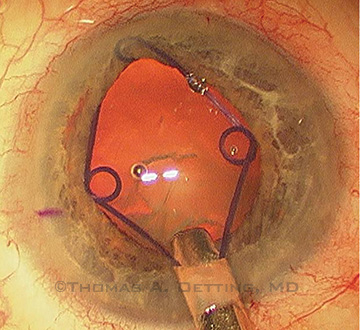
A Malyugin ring may be used to enlarge a small pupil such as those seen in cataract patients who previously underwent panretinal photocoagulation for diabetes.
|
Eyes With AMD
“There is increasing evidence that the risk for worsening of preexisting AMD following cataract surgery is low,” the Academy’s 2011 update of the Preferred Practice Pattern: Cataract in the Adult Eye concluded.1 Moreover, recent evidence from the Age-Related Eye Disease Study (AREDS) indicates that vision improves across the spectrum of AMD severity.2
Wet AMD. In cases of ongoing treatment of proliferative AMD, Dr. Scott recommends choosing the date for the phacoemulsification surgery based on two criteria: 1) the patient’s response to intravitreal injections of an anti– vascular endothelial growth factor (VEGF) drug, and 2) the date of the most recent injection.
“I prefer to have the patient not get his or her cataract removed until we get to a point where I feel we have a good handle on the disease, and the patient is responding well,” she said.
The surgery should be timed to take advantage of the anti-VEGF drug’s inflammation-dampening properties, Drs. Scott and Oetting said. “I try to time my intravitreal injections to be about one week prior to the cataract surgery, so the anti-VEGF agent can be working while the eye is in the postoperative period,” Dr. Scott said. And Dr. Oetting said he is comfortable scheduling the surgery up to two weeks after the intravitreal injection.
In contrast, Kimberly A. Drenser, MD, PhD, of Royal Oak, Mich., said, “Usually, I will try to treat them before their cataract surgery and then bring them back a month later for an exam and possible retreatment. However, patients undergoing treatment for wet AMD can get their injections anytime, even as soon as the day after cataract surgery.”
Dry AMD. Though they may have lost central vision, patients with the dry form of AMD can benefit from having their cataracts removed, Dr. Scott said. “I have seen many patients with pretty severe loss of central vision who really appreciate the brightness of their peripheral vision after cataract surgery.”
Even so, these patients should be cautioned that their visual prognosis remains guarded, Dr. Scott said. “These are the patients with whom I particularly would have a long discussion about the ultimate visual outcome. You have to be very clear that they won’t get back their lost central vision.”
More on informed consent. “The biggest mistake I see general ophthalmologists making is not discussing the macular degeneration in enough depth relative to the cataract surgery prior to doing the surgery,” Dr. Drenser said. “The patients then are upset afterward because they don’t understand that the cataract is different from the macular degeneration.” This holds true in cases of both wet and dry AMD, she said.
If undiagnosed AMD is suspected, spectral-domain optical coherence tomography (SD-OCT) can facilitate a diagnosis and allow referral for treatment before the cataract surgery, Dr. Drenser said (see “A Note on the Role of OCT”).
Dr. Drenser also recommends sending patients in whom AMD is known or suspected to a vitreoretinal specialist for exam prior to surgery. This will reassure the patient, and taking this step will strengthen the informed consent. “It’s a matter of the patient knowing that there may be a postoperative problem or that they might not have the vision that they were expecting because of the macular degeneration,” she said. And if the AMD is not noted before cataract surgery takes place, patients “will associate the surgery with the progression of their macular degeneration,” she warned.
Reduce Risk of Postoperative Edema
Cystoid macular edema (CME) is one of the most common ways in which a retinal, or another, ocular comorbidity can complicate the course of recovery from phacoemulsification and lens replacement. A number of retinal conditions—including diabetic retinopathy, retinitis pigmentosa, and vascular occlusion—are known to increase the risk of postoperative CME, even after uneventful cataract surgery.1 Moreover, complicated cataract surgery, especially with vitreous loss, increases the risk of postoperative CME, Dr. Oetting said.
Estimating risk. The overall incidence of clinically significant CME after cataract surgery has been reported to be between 1 and 3 percent in various studies. However, a 2007 study found that retinal vein occlusion and epiretinal membrane (ERM) significantly increased the patient’s risk of developing CME after uncomplicated cataract surgery.1
In that study, 2.4 percent of subjects developed postoperative CME, mostly within three months of the cataract surgery, the researchers found. But patients with a history of retinal vein occlusion were 31.75 times more likely to develop CME; for those with ERM, the relative risk factor was 4.93.1
Need for prophylaxis. The 2007 study concluded that treating high-risk patients with NSAIDs after cataract surgery decreases the incidence of postoperative CME to that of patients who are not at high risk.1 Although this assessment has won wide clinical acceptance, prophylaxis regimens vary from one surgeon to another, Dr. Oetting said.
“There isn’t really good evidence about how long to use an NSAID or [even] which drug to use,” Dr. Oetting said.2 Both he and Dr. Scott prefer ketorolac 0.5 percent, an inexpensive drug that is known to be an effective treatment for chronic CME.
Recommended protocol. Dr. Oetting uses the following regimen for CME prophylaxis in cataract patients who are particularly at risk of developing postoperative edema.
- One week before surgery. Begin using a topical NSAID. Dr. Oetting prescribes ketorolac 0.5 percent (one drop, four times per day).
- Day of surgery. Discontinue the NSAID until one week postop to avoid epitheliopathy of the healing cornea. Dr. Oetting typically does not inject intraoperative steroids.
- Postop day 1. Begin a topical corticosteroid eyedrop such as prednisonole acetate 1 percent (one drop, four times per day) and continue with taper for at least one month. If CME develops, the rate is increased back to four times a day until the CME clears. (The patient also uses a topical antibiotic drop during the first postop week.)
- One week after surgery. Resume the topical NSAID as before. Continue daily doses for one to two months, depending on the physician’s assessment of the risk profile—for instance, eyes with minor ERM or with no history of diabetic macular edema (DME) would receive treatment for one month, while those with previous DME, CME in the other eye, or uveitis would be treated for two months.
___________________________
1 Henderson BA et al. J Cataract Refract Surg. 2007;33(9):1550-1558.
2 Yilmaz T et al. Eye (Lond). 2012;26(2):252-258. |
Eyes With Retinal Vein Occlusion
Because the risk of cystoid macular edema (CME) increases by a factor greater than 30 if there is a previous retinal vein occlusion (RVO), the experts recommend performing prophylaxis against CME. Dr. Scott prescribes her preferred topical nonsteroidal antiinflammatory drug (NSAID), ketorolac 0.5 percent, for RVO patients to use for three days before surgery and then resumes the NSAID one week after surgery. Dr. Oetting’s NSAID regimen is similar and continues for one to two months (see “Reduce Risk of Postoperative Edema”).
Eyes With Epiretinal Membrane
An eye with moderate cataract and an epiretinal membrane (ERM) demands strong presurgical diagnostic and decision-making skills, Dr. Scott said. As she explained, the surgeon faces a two-part question: 1) Which surgery—cataract or membrane peeling—should be performed first? 2) Alternatively, should a combined procedure be performed?
To answer this, the surgeon must consider both the patient’s visual history and the information gleaned from SD-OCT, the experts said.
What the history reveals. “If the patient’s main complaint is distortion in his or her vision, that tells me that the ERM is causing a significant portion of the vision loss,” Dr. Scott said. In contrast, if the patient’s main complaint is blurred vision, “then that tells me that the cataract may be playing more of a part. These things are not absolute, though.”
What the OCT clarifies. Once the patient’s history is correlated with OCT images, the surgical path should become clearer. For instance, Dr. Scott said, “If I can see that the ERM is disturbing the natural foveal contour on OCT, then I would move toward having a combined surgery.”
However, “If there is an ERM but the foveal contour isn’t particularly distorted, then my thoughts are that most of the blurry vision is not retinal in origin, and the membrane peeling can be done later,” she said.
A Note on the Role of OCT
When in doubt, use SD-OCT to detect early signs of a comorbidity that cannot be seen during a clinical exam and to guide surgical decisions, the experts said.
“There’s still no substitute for a good retina specialist. But OCT has been the great equalizer for the retinal exam. It makes all of us halfway-good at the retinal exam because we can see such detail,” Dr. Oetting said. For instance, an image might reveal subtle macular thickening from early CME or subclinical choroidal neovascularization in a patient who was thought to have dry AMD.
Moreover, OCT images can prove helpful in talking with patients and their families, Dr. Oetting said. “The beauty of the OCT is that it’s colorful, and it’s obvious to anyone looking at it that there’s something funny there. You can show them an OCT of a normal retina and then show them theirs,” Dr. Oetting said. “You even can run another OCT after the cataract surgery and show them [the outcome].”
___________________________
1 Cataract in the Adult Eye. San Francisco: American Academy of Ophthalmology; 2011.
2 Age-Related Eye Disease Study 2 Research Group. Ophthalmology. 2014;121(6):1229-1236.
___________________________
Kimberly A. Drenser, MD, PhD, is clinical professor of ophthalmology at the William Beaumont Oakland University College of Medicine and a partner in Associated Retinal Consultants, both in Royal Oak, Mich. Financial disclosure: Has equity interest in Focus-ROP and Retinal Solutions and has received lecture fees from ThromboGenics.
Thomas A. Oetting, MD, is professor of clinical ophthalmology and director of the ophthalmology residency program at the University of Iowa and chief of eye service and deputy director of surgery service at the Veterans Administration Medical Center in Iowa City. Financial disclosure: None.
Adrienne Williams Scott, MD, is assistant professor of ophthalmology at the Wilmer Eye Institute in Baltimore. Financial disclosure: None.
Further Reading
Goldman JM, Karp CL. Adjunct Devices for Managing Challenging Cases in Cataract Surgery: Pupil Expansion and Stabilization of the Capsular Bag. Curr Opin Ophthalmol. 2007;18(1):44-51.
Goldman JM, Karp CL. Adjunct Devices for Managing Challenging Cases in Cataract Surgery: Capsular Staining and Ophthalmic Viscosurgical Devices. Curr Opin Ophthalmol. 2007;18(1):52-57.
Kim SJ, Bressler NM. Optical Coherence Tomography and Cataract Surgery. Curr Opin Ophthalmol. 2009;20(1):46-51.
Loewenstein A, Zur D. Postsurgical Cystoid Macular Edema. Dev Ophthalmol. 2010;47:148-159.
Oetting TA. Cataract Surgery for Greenhorns. |