Download PDF
For many years, patients with ocular surface disease—whether from traumatic, immunologic, or genetic causes—lacked satisfactory therapeutic options. Over the past decade, however, much progress has been made in understanding how to replenish damaged cells and tissue.
Today, a variety of stem cell approaches can address mild to severe limbal stem cell deficiency (LSCD), a condition that often leads to corneal vascularization and opacification and, ultimately, vision loss (Fig. 1). And more solutions are on the way.
“We can provide a reasonable opportunity for visual recovery for the vast majority of patients with LSCD, even those who wouldn’t have even been referred just 15 to 20 years ago,” said Edward J. Holland, MD, director of cornea services at the Cincinnati Eye Institute and professor of ophthalmology at the University of Cincinnati. “We’re far ahead in ocular surface stem cell transplantation because solutions are easier to come by” than they are in retinal disorders or glaucoma. Not only is the cornea a less complex structure with a supply of limbal stem cells, but also, “We get multiple chances to fix it,” he said. If there is scarring or failure of a retinal procedure, for instance, that’s it. “But with cornea,” he said, “we can go back and try again.”
|
Worst-Case Scenario
|
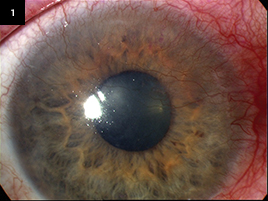 |
|
Limbal stem cell failure following treatment for ocular surface squamous neoplasia. The hallmark of total stem cell failure— "conjunctivalization" of the cornea—is evident.
|
Defining success with ocular surface stem cell transplants isn’t always straightforward. But it traditionally includes improvements in vision, comfort, and the ocular surface, as well as a reduction in corneal vascularization, said Stephanie Watson, MBBS, PhD.
Although it’s long been known that the corneal epithelium contains adult tissue stem cells that renew and repopulate it, what cornea surgeons have come to appreciate more recently is the importance of the specialized microenvironment in which they reside: the stem cell niche.
Surgical tips for ensuring success with the niche include the following.
- First, clear the damage. If the niche is compromised, the stem cell transplant will be, too, said Dr. Watson, clinical professor of ophthalmology and eye health at the Save Sight Institute at the University of Sydney, Australia. “Many grafts fail due to problems such as scarring,” she said, adding that a more global repair of the eye’s front surface can help prevent this.
- Next, be a good gardener. Like a garden dependent on watering and good soil, the stem cell niche also needs tending, said Dr. Watson. This includes optimizing the lids and tear film, controlling inflammation and vascularization, and avoiding the toxic effects of other topical medications, such as those used to treat glaucoma.1
Working With Current Techniques
A variety of adult corneal stem cell autografts and allografts are the current mainstay of limbal stem cell transplantation. These procedures include conjunctival limbal autograft (CLAU), which involves conjunctival and limbal tissue from the fellow eye; living-related conjunctival limbal allograft (lr-CLAL), which involves conjunctival and limbal tissue from a living relative; and keratolimbal allograft (KLAL), which involves limbal stem cells from the peripheral cornea of a cadaver.2
Patient selection. Preoperative conditions dictate which procedure is best, said Dr. Holland. For instance, a patient with a healthy conjunctiva and exclusively limbal disease—possibly from congenital aniridia or a chemical injury—might not have a good family match. “This patient needs limbal stem cells, and we have no reluctance about using an unmatched cadaver donor.”
Combining procedures. Patients with severe conjunctival disease, such as Stevens-Johnson syndrome or mucous membrane pemphigoid, don’t do well with a KLAL alone, he said. They might get a conjunctival allograft or a procedure recently pioneered by Dr. Holland and his colleagues. This approach, a combined lr-CLAL/KLAL, transplants both living-related conjunctiva and limbus and keratolimbal stem cells.3 “We’ve found it very successful for patients with both conjunctival and limbal disease.”
Sometimes limbal stem cell surgery must be combined with other forms of corneal surgery, such as penetrating keratoplasty, to achieve the optimal visual outcome, Dr. Watson said. “That’s because stem cell failure can damage other parts of the cornea.”
Model organ transplants. Dr. Holland and his colleagues have had good success with allografts, he said, even in cases of severe ocular surface disease. They’ve accomplished this by adopting “a program to parallel what’s done in organ transplantation programs, something many ophthalmologists and cornea specialists have been reluctant to embrace due to a fear of immunosuppression,” said Dr. Holland. This fear may be misplaced, given certain statistics. Publishing on a decade of immunosuppression in more than 150 patients undergoing more than 200 ocular surface transplants, Dr. Holland and his colleagues have reported no deaths and only three major adverse effects—all due to preexisting problems.
Dr. Holland’s group uses human leukocyte antigen (HLA) typing, which is critical in finding more compatible tissue to transplant. In addition, they employ a team approach, with the team consisting of a “quarterbacking” cornea surgeon; a transplant nurse coordinator, who oversees screening, medication, and side effects; an internal medicine specialist with expertise in immunosuppressive medication; and other ophthalmologists as needed, such as glaucoma subspecialists.
No transplant? Some patients, such as those who are older and have multiple medical problems, may not be good candidates for ocular surface stem cell transplants at all, said Dr. Holland. Instead, they may do better with a keratoprosthesis.
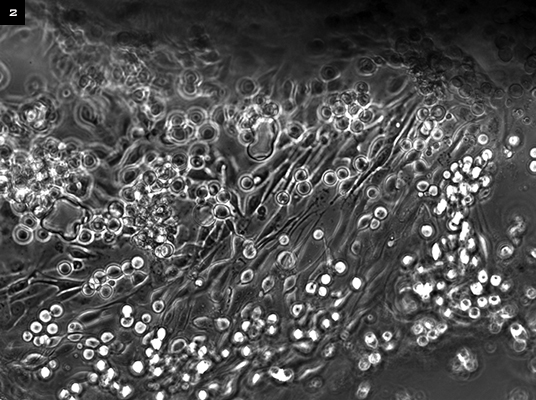 |
|
EX VIVO OPTION. In an entirely autologous approach, cells are retrieved from the fellow eye and grown on a contact lens. The lens is later applied to the eye after damaged tissue is removed.
|
Ex Vivo Expansion Emerges
Ex vivo expansion of autologous cells to reconstruct the ocular surface is one of the more promising techniques to come along in recent years, said Dr. Watson. “Prior to its use, we were doing transplants with a success rate of about 45 percent. With ex vivo cultures, the success rate is 75 to 80 percent.”
Early outcomes. Pellegrini and Rama have extensive expertise using these adult stem cell culture techniques for unilateral ocular burns and have reported on a series of more than 100 patients with over 10 years of follow-up, Dr. Watson said. More than three-quarters of the patients experienced permanent restoration of a transparent, renewing corneal epithelium, with eyes remaining stable over time.4
Totally autologous. Dr. Watson and her colleagues have pioneered an approach using ex vivo expansion of cells retrieved from the fellow eye and grown on an FDA-approved contact lens, which is applied to the eye surface after damaged tissue is removed (Fig. 2).
The technique is 100 percent autologous, she said, because cells are grown in the patient’s own serum instead of using an amniotic membrane or mouse feeder cells. The lens minimizes the risk of infection and is readily available, unlike an amniotic membrane, said Dr. Watson.
She and her colleagues are writing up trial results, having waited a year to confirm the cells’ status in 16 patients. Early results are promising. “The first three who were treated beginning in 2007—one with aniridia and two with melanoma damage—all maintained their ocular surface,” she said.
New cell sources. Cells for ex vivo expansion typically originate from the fellow eye or a living-related donor. “And for patients who have bilateral disease,” said Dr. Watson, “we’ve also used conjunctiva, since there’s lots of research that supports [the hypothesis that] it contains stem cells.”
Stem cell researchers have also used epithelial cells from the oral mucosa, thus providing another potential option for autologous transplant to reduce the risk of rejection.5
“Although those cells have already been differentiated, the oral mucosa does not supply corneal epithelium,” said Dr. Holland, adding that the ultimate goal might be to take mesenchymal stem cells from bone marrow and prompt them to differentiate into corneal epithelium. “But we’re years away from that.”
Standard of care. “I’m excited about some of these techniques because they may eventually get us away from immunosuppression,” said Dr. Holland. “But right now, I don’t think any of the ex vivo procedures provide the same success we have [had] in patients with severe ocular surface disease. It’s our belief that, at the present time, the procedure to get the best visual acuity long term is one of the standard techniques.”
Update on Human Embryonic Stem Cells
Swedish scientists recently succeeded in culturing epithelial cells on damaged human corneas in vitro using human embryonic stem cells (hESCs).1
Road to success. Using time-lapse imaging, the scientists first grew embryonic stem cells in a special medium, where they started to develop into the desired niche, said Ulf Stenevi, MD, PhD, professor and chairman of ophthalmology at the University of Gothenburg’s Sahlgrenska Academy in Sweden. “After 10 days to three weeks, we asked, ‘Are these really epithelial cells?’” he said. “We then put these cells onto the human cornea that was lacking epithelial cells and said, ‘Please develop into corneal epithelium.’ After about three years and many tries, we finally came up with the right ‘recipe.’ It behaves like the developing corneal epithelium.”
More work to do. The scientists started their work with epithelial cells, which have many markers and are easier to observe, said Dr. Stenevi. They are now working on prompting embryonic stem cells to develop into other types of cells, for which there is a greater demand.
Though hESCs have the lure of limitless supply and potential solutions for the endothelium, actual clinical application in patients is a bit distant, he said. Much work is required to develop the technique and to ensure that the stem cells develop into correct cell types and not into something harmful, such as tumors, he added.
___________________________
1 Hanson C et al. Acta Ophthalmol. 2012 Jan. 26. doi:10.1111/j.1755-3768.2011. 02358.x. [Epub ahead of print.]
|
Remaining Challenges
Managing costs. Clinical challenges are sometimes less daunting than those of cost, documentation, and regulatory hurdles, said Dr. Watson. For instance, in the case of allografts, said Dr. Holland, insurance doesn’t cover the cost of HLA testing for a potential donor. Medications are also expensive, he said, but insurance coverage has come a long way on this point.
And certifying a lab to meet regulatory requirements for ex vivo expansion and maintaining it to culture cells over a 10-day period is expensive, said Dr. Watson. To defray costs, she said, labs in Australia and New Zealand are developing a collaborative network for patients with stem cell failure.6
Rescuing cells. In the case of chemical burns, more research is needed to see what can be done early on to rescue stem cells that are injured, rather than putting in new ones, Dr. Watson said.
Enriching transplants. “We need to enhance techniques to ensure we’re transferring an optimal number of stem cells,” said Dr. Watson. She added that identifying stem cells can be a challenge—and that when collecting cultures, you don’t select stem cells alone. “Some are ‘daughter cells,’ so you need to enrich the transplant to ensure a higher percentage of stem cells.”
Ensuring cell survival. As for ex vivo expansion, how long will cells survive? asked Dr. Holland. Moreover, does this technique result in the same amount of ocular surface tissue as seen with standard transplants? “These are all questions that still remain.”
___________________________
Drs. Holland, Stenevi, and Watson report no related financial interests.
___________________________
1 Tseng SC, Tsubota K. Am J Ophthalmol. 1997;124(6):825-835.
2 Holland EJ, Schwartz GS. Cornea. 1996;15(6):549-556.
3 Biber JM et al. Cornea. 2011;30(7):765-771.
4 Rama P et al. N Engl J Med. 2010;363(2):147-155.
5 Ang LP et al. Arch Ophthalmol. 2006;124(11):1543-1551.
6 Harkin DG et al. Clin Experiment Ophthalmol. 2012 Sept. 7. [Epub ahead of print.]