Download PDF
Leukocoria, or “white pupil,” is one of the primary signs of retinoblastoma. However, a number of other conditions may also present with leukocoria, and it is critical to differentiate retinoblastoma from these so-called pseudoretinoblastomas for proper management.1 Shields and coworkers have reported 27 different pseudoretinoblastoma conditions.2 Some of the most common include
- Coats disease
- Persistent fetal vasculature (PFV)
- Ocular toxocariasis
- Familial exudative vitreoretinopathy (FEVR)
- Retinopathy of prematurity (ROP)
- Astrocytic hamartoma
- Vitreous hemorrhage
- Coloboma
- Endogenous endophthalmitis
- Rhegmatogenous retinal detachment
Leukocoria is often first noticed by family members or on flash photography, and such images can be helpful to the ophthalmologist. However, red eye removal software in modern cameras may confound the detection of leukocoria. Also, if the subject in the photo is looking approximately 15° off axis nasally, the optic disc shadow can fill the pupil, giving a white reflex.
Any patient with an abnormal red reflex should be evaluated promptly by an ophthalmologist. A red reflex exam is also warranted at every pediatric visit from birth to 3 years and, later, as part of vision screening.
A detailed history, a good ophthalmic exam, and ancillary tests are essential in evaluating patients with leukocoria. This article outlines a practical, stepwise approach to identifying key diagnostic findings in retinoblastoma and pseudoretinoblastomas.
 |
|
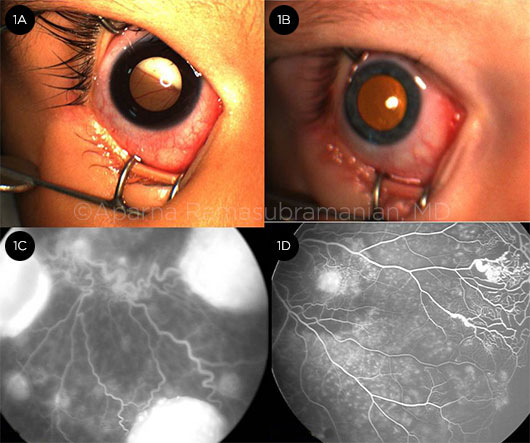
RETINOBLASTOMA VS. COATS. (1A) Leukocoria in retinoblastoma; prominent vessels dip into the retinal detachment. (1B) Xanthocoria (yellow pupil) in Coats disease due to retinal exudation. (1C) FA in retinoblastoma shows dilated and tortuous vessels feeding the hyperfluorescent tumor compared with (1D) peripheral telangiectasia and capillary nonperfusion in Coats disease.
|
History
Although a detailed history is important to provide diagnostic clues, it is not reliable in isolation and must be correlated with findings from the clinical examination. The following topics should be explored.
I. History of Present Illness
A. Age at onset: The average age at diagnosis of retinoblastoma is 18 months, and the average age for Coats disease is 5 years.
B. Duration of the abnormal white reflex: A review of family photographs can help ascertain if and when the reflex changed. A previously normal red reflex decreases the likelihood of a congenital disease such as PFV and suggests an acquired etiology.
C. Other symptoms, including pain, redness, photophobia, strabismus, and blurred vision, should be noted.
II. Past Ocular History
A. History of retinopathy of prematurity: ROP can present as a white pupil due to retrolental fibrous tissue and total retinal detachment.
B. Trauma: Ocular trauma can lead to cataract, retinal detachment, or vitreous hemorrhage, any of which can produce an abnormalred reflex.
III. Medical History
A. Prematurity
B. Arthritis: Juvenile inflammatory arthritis (JIA) can cause dense uveitis that mimics retinoblastoma, especially diffuse infiltrating retinoblastoma.
C. Prenatal infections: TORCH syndrome (which may include toxoplasmosis, other agents, rubella, cytomegalovirus, and herpes simplex) may show a white reflex.
D. Birth trauma
E. Exposure to pets: Consider toxocariasis (if child is exposed to puppies) or toxoplasmosis (cats).
F. Presence of skin lesions: Incontinentia pigmenti can cause skin lesions ranging from vesicles to hyperpigmentation to atrophy. It may be associated with retinal detachment resulting in leukocoria.
G. Other systemic diseases: Tuberous sclerosis (associated with retinal astrocytomas), endogenous endophthalmitis.
IV. Family History. Several conditions that produce leukocoria have a hereditary component.
A. Retinoblastoma: Autosomal dominant with incomplete penetrance (approximately 90%), although only 10% of patients with retinoblastoma have a family history.
B. Familial exudative vitreoretinopathy: FEVR has an autosomal dominant inheritance pattern, though many patients may be asymptomatic.
C. Coloboma: Autosomal dominant inheritance of mutation in PAX6 gene on chromosome 11 has been noted.
 |
|
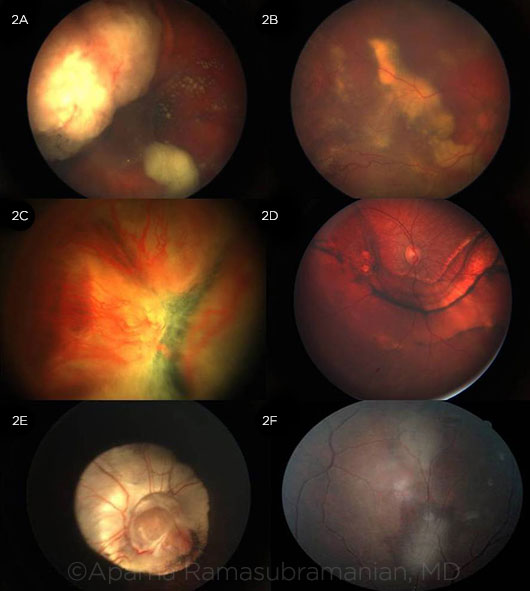
FUNDUS APPEARANCE. (2A) Endophytic retinoblastoma with vitreous seeds can be differentiated clinically from (2B) Coats disease, showing exudation, telangiectasia, retinal detachment, and macrocyst. (2C) Total retinal detachment with prominent vasculature in PFV can be differentiated from other causes of retinal detachment, of which trauma (2D) is the most common. (2E) Large optic disc and chorioretinal coloboma can cause leukocoria. (2F) Astrocytic hamartoma in tuberous sclerosis.
|
Clinical Examination
A thorough clinical examination is pivotal to the diagnosis of leukocoria. Following are important clues for distinguishing among conditions.
I. Laterality
A. Unilateral disease: Retinoblastoma (60%), Coats disease, PFV, toxocariasis, vitreous hemorrhage, retinal detachment.
B. Bilateral disease: Retinoblastoma (40%), FEVR, ROP, astrocytic hamartoma, endogenous endophthalmitis. However, Coats disease may be bilateral in patients with facioscapulohumeral dystrophy, and there have been rare reports of bilateral PFV.
II. Color of the Reflex
A. White pupillary reflex is typical of retinoblastoma (Fig. 1A).
B. Yellow pupillary reflex, or xanthocoria, from exudates and exudative retinal detachment is indicative of advanced stages of Coats disease (Fig. 1B).
C. Blue-gray pupil is commonly seen in congenital cataracts.
III. Visual Acuity. It is useful to document the visual acuity in verbal children. In preverbal children, Teller visual acuity measurements can be obtained.
IV. Intraocular Pressure. IOP may be elevated in both retinoblastoma and Coats disease secondary to anterior segment neovascularization. The IOP can also be elevated in JIA uveitis due to trabeculitis.
V. Pupil. It is important to note the presence of afferent pupillary defect, which may be a poor prognostic sign.
VI. Strabismus. In about 20% of retinoblastoma cases, strabismus is the presenting feature. It may also be associated with other diseases that decrease vision, leading to interruption of the fusional mechanism.
VII. Anterior Segment
A. PFV: Associated findings include microphthalmia, microcornea, shallow anterior chamber, persistent tunica vasculosa lentis, cataract, fine vessels coursing over the iris to the anterior lens surface, and retrolental fibrovascular membrane.
B. Anterior chamber retinoblastoma: May appear as white, fluffy seeds on the iris stroma or layered as pseudohypopyon.
C. Ciliary body medulloepithelioma: Signs include lens coloboma, cataract, glaucoma, and retrolental mass.
D. Coats disease: Anterior chamber cholesterolosis with free-floating yellow crystalline cholesterol deposits in the aqueous.
E. Iris coloboma: Can be associated with choroidal coloboma.
F. Anterior chamber inflammation: Consider JIA uveitis and endogenous endophthalmitis.
G. Neovascularization of iris and glaucoma: Can be seen in both retinoblastoma and Coats disease and can also be associated with any long-standing retinal detachment.
VIII. Fundus
A. Vitreous: In Coats disease, the vitreous remains clear; while vitreous seeds are present in endophytic retinoblastoma. There may be vitritis in toxocariasis and a persistent hyaloid canal in PFV.
B. Optic disc: The funnel-shaped excavated optic nerve head of morning glory disc or coloboma (Fig. 2E) may cause leukocoria. In PFV, fundus findings may include Bergmeister papilla, a retinal fold from the disc to the periphery, hypoplastic or dragged macula, hypoplastic optic nerve, or tractional retinal detachment with a stalk to the optic disc.
C. Retinal vessels: In retinoblastoma, the vessels are uniformly dilated and tortuous (Fig. 1C), but in Coats disease, the vascular dilation is irregular with saccular enlargement (Fig. 1D), and there may be peripheral telangiectasia. In retinoblastoma, retinal vessels dip into the detachment (1A), unlike Coats disease, in which they course over the detachment (2B). Also, peripheral dragging from fibrovascular proliferation is seen in FEVR.
D. Retina:
1. Retinoblastoma can present in 3 growth patterns: exophytic, which leads to retinal detachment (Fig. 1A); endophytic (Fig. 2A) with vitreous seeding; and the rare diffuse infiltrating type, which grows along the retinal layers, without causing retinal elevation, and mimics uveitis.
2. Coats disease presents as retinal exudation and exudative retinal detachment (Fig. 2B). Macular exudation may mimicbretinoblastoma—differentiating features include irregular lightbulb telangiectasia in the peripheral fundus and yellow subretinal and intraretinal exudation. A subretinal gliotic nodule in Coats disease can be mistaken for a solitary retinoblastoma lesion. However, there is no feeder vessel or tortuous draining vein as in retinoblastoma.
3. PFV is usually unilateral with a central fibrovascular stalk emanating from the disc, often with retinal detachment (Fig. 2C).
4. Ocular toxocariasis causes a retinal or subretinal granuloma that can mimic exophytic retinoblastoma. Chronic toxocariasis endophthalmitis presents as severe granulomatous vitritis with cyclitic membrane, retinal detachment, leukocoria, and hypopyon.
5. FEVR is characterized by peripheral retinal nonperfusion with resultant neovascularization, retinal traction, and fibrovascular proliferation leading to retinal detachment.
6. ROP also presents with abnormal vascularization leading to fibrosis and retinal detachment.
7. Astrocytic hamartoma in tuberous sclerosis presents as a flat (Fig. 2F) or elevated lesion. It can be differentiated from retinoblastoma by its lack of retinal detachment, lack of growth, and the course of retinal blood vessels under or around the astrocytic tumor.
8. Vitreous hemorrhage can be secondary to trauma, posterior uveitis, or any of the vascular abnormalities noted above. Careful examination of the underlying retina will point toward the diagnosis.
9. Uveal coloboma appears as a sharply demarcated, glistening white, bowl-shaped excavation in the fundus (Fig. 2E). Unlike retinoblastoma, no elevated mass is seen.
10. Endogenous endophthalmitis presents as anterior and posterior segment inflammation and there may be signs of systemic sepsis. Cultures may be confirmatory.
11. Retinal detachment from any other etiology (e.g., trauma, Fig. 2D) needs to be differentiated from retinoblastoma. Thorough fundus examination evaluating for a mass lesion or retinal or vitreous seeds is critical.
 |
|
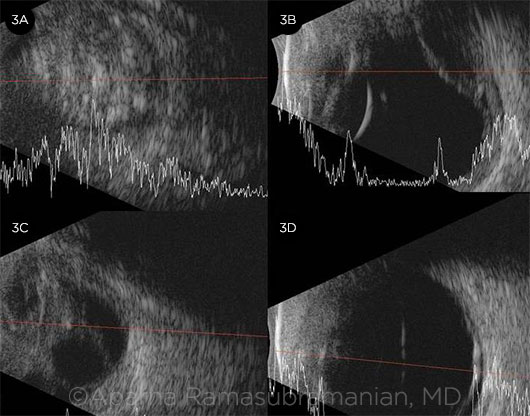
ULTRASONOGRAPHY. (3A) Mass in the vitreous cavity with multiple dense calcifications is suggestive of retinoblastoma. (3B) Exudative retinal detachment with linear calcification due to osseous metaplasia can be seen in Coats disease. (3C) Retinal detachment with a stalk is seen in PFV. (3D) Retinal detachment secondary to trauma, with no other abnormalities.
|
Ancillary Tests and Imaging
I. Ultrasonography. We recommend performing ultrasonography in all patients with leukocoria, as it is inexpensive, noninvasive, and highly specific for detecting the calcifications distinctive of retinoblastoma (seen in 90% of cases). Following are characteristic findings.
A. Retinoblastoma: A solid retinal mass with high-intensity internal echoes due to intratumoral calcification (Fig. 3A, Web Extra).
B. Coats disease: Subretinal fluid and retinal detachment without a solid retinal mass (Fig. 3B, Web Extra). Occasionally, a retinal macrocyst may be seen. Rarely, there may be linear dystrophic retinal calcifications from chronic retinal detachment at the level of RPE, unlike retinoblastoma, where the calcification is within the intraocular tumor.
C. PFV: Persistent hyaloid remnants in PFV (Fig. 3C, Web Extra).
D. Ocular toxocariasis: Absence of calcification in the elevated granulomas.
E. Medulloepithelioma: Ultrasound biomicroscopy is useful to demonstrate multicystic irregular internal reflectivity of the ciliary body.
F. Retinal detachment: In patients with retinal detachment (Fig. 3D, Web Extra), ultrasonography can be useful to evaluate for the presence of a mass.
G. Astrocytic hamartoma: Calcifications may be present in astrocytic hamartoma, but they are glistening yellow rather than the dull, chalky white seen in retinoblastoma.
II. Fluorescein Angiography. FA with Retcam photography is useful in evaluating children with leukocoria. In Coats disease, FA shows retinal telangiectasia manifesting as “lightbulbs” with late leakage from abnormal vessels and peripheral nonperfusion (Fig. 1D). Retinoblastoma is associated with a relatively rapid homogeneous hyperfluorescence without retinal dragging (Fig. 1C), while granulomas due to toxocariasis demonstrate reticular hyperfluorescence and are commonly associated with retinal traction. A peripheral avascular zone is seen in ROP and FEVR.
III. Optical Coherence Tomography. OCT is increasingly used to evaluate macular tumors or simulating lesions. Noninvasive and well tolerated, it provides invaluable information on the presence of fibrosis, edema, or subretinal fluid in the macula.
IV. Computerized Tomography. CT is generally avoided in children because of radiation risks but can be considered if the presence of calcification is questionable. Ultrasonography has good sensitivity to detect calcification; thus, CT is very rarely required.
V. Magnetic Resonance Imaging. MRI is performed to evaluate the pineal gland and to visualize the optic nerve and choroid to detect infiltration in patients with retinoblastoma. Retinoblastoma appears isointense to hyperintense on T1 and hypointense on T2. Coats disease appears as a hyperintense subretinal exudate. Ocular toxocariasis appears as an isointense granuloma on T1 and hyperintense on T2.
VI. Blood Work. Toxocara exposure is common, so a positive serologic test is supportive but not diagnostic. Blood work is also useful to diagnose TORCH infections and to identify the underlying disease in patients with endogenous endophthalmitis.
VII. Genetic Testing. This is important in diseases such as retinoblastoma, FEVR, and astrocytic hamartoma, both for confirming the diagnosis and for genetic counseling. Newer studies have indicated a somatic mutation in the NDP gene (mutant in Norrie disease) in Coats disease.
VIII. Fine-Needle Aspiration Biopsy. FNAB is avoided in patients suspected of having retinoblastoma due to the risk of metastasis. If retinoblastoma is ruled out, intravitreal tap may be useful in eyes with endogenous endophthalmitis to make the diagnosis or in patients with chronic endophthalmitis due to toxocariasis in which aqueous humor cytology will reveal eosinophils.
___________________________
1 Kaliki S, Shields CL. Differential diagnosis of retinoblastoma. In: Ramasubramanian A, Shields CL, eds. Retinoblastoma. New Delhi, India: Jaypee Brothers Medical Publishers; 2012;46-60.
2 Shields CL et al. Ophthalmology. 2013;120(2):311-316.
___________________________
Dr. Syed is an ophthalmology resident, and Dr. Ramasubramanian is a pediatric ophthalmologist and an ocular oncologist; both are at University of Louisville, Kentucky. Relevant financial disclosures: This work was supported in part by an unrestricted educational grant from Research to Prevent Blindness, New York.