Download PDF
Sturge-Weber syndrome (SWS) belongs to a group of disorders known as phakomatoses. These disorders are characterized by hamartomas, which are congenital tumors arising from tissue that is normally found at the involved site. Unlike other phakomatoses, SWS has no hereditary pattern and is caused by a somatic mutation in the GNAQ gene.1 The incidence of this condition is approximately 1 per 50,000 live births, with no reported racial or gender predilection. The classic triad of SWS consists of facial cutaneous venous dilation, often called port-wine stain (PWS), leptomeningeal capillary-venous malformation, and ocular abnormalities. This review discusses the clinical features of SWS with an emphasis on secondary glaucoma, the most common ocular manifestation of this disorder.
Cutaneous Features
PWS is typically present on the forehead and upper eyelid, along the ophthalmic and maxillary divisions of the trigeminal nerve. Although usually unilateral and apparent at birth, the lesion may extend to both sides of the face and extremities. In newborns, PWS is flat with a light pink appearance. With increasing age, it becomes darker red or deep purple with hypertrophy and nodularity of the associated vascular ectasias. The ipsilateral nasal and buccal mucosa may also be involved. The current management of PWS is treatment with a pulsed dye laser. Usually, early intervention and multiple treatments are required to lighten the skin discoloration and enhance cosmetic outcomes.
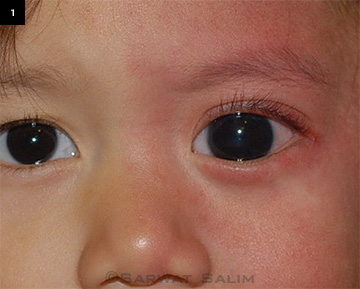 |
|
EXTERNAL FINDINGS. Cutaneous venous dilation, or port-wine stain, on the left side of the face with buphthalmos and enlarged cornea.
|
Neurological Features
Intracranial vascular anomalies. Leptomeningeal capillary-venous malformation occurs in 10 to 20 percent of newborns with a typical PWS.2 However, the risk of intracranial lesions increases with the size and bilateral presence of the PWS. The central nervous system (CNS) is involved in about 10 percent of patients without an accompanying facial lesion. In such cases, patients develop neurological symptoms later in childhood or adulthood, and the diagnosis may be missed unless appropriate neuroimaging is performed.
The leptomeningeal vascular malformation typically occurs on the same side as the PWS and commonly affects the parietal and occipital areas. The CNS lesions usually progress over time, and chronic tissue ischemia caused by venous stasis results in corresponding dystrophic calcification. The characteristic radiographic appearance of cortical venous calcification is parallel double densities, or “railroad tracks.”
Epilepsy. The most common neurologic complication of SWS is epilepsy, which occurs in 75 percent and 95 percent of patients with unilateral or bilateral brain involvement, respectively. Seizures usually occur within the first year of life but may appear at any age. These may manifest with any acute illness or may accompany the hemiparesis that occurs contralateral to the site of CNS involvement. Seizures may be focal motor, complex partial, or atonic. Permanent hemiplegia and homonymous hemianopia may develop as a consequence of epilepsy.
Seizures may be controlled with anticonvulsant therapy in 40 percent of patients. Refractory cases may benefit from surgery, including hemispherectomy or lobectomy, although outcome data are scarce. Onset of seizures early in life and poor response to anticonvulsant therapy increase the risk of cognitive impairment.
Other CNS complications. Developmental delay, learning disabilities, and severe headaches commonly occur in children with SWS. These children also experience social and behavioral difficulties. Mental retardation may occur in up to 60 percent of all patients with SWS.
Ocular Features
Vascular abnormalities of the eyelid, orbit, conjunctiva, episclera, ciliary body, retina, and choroid may occur. Dense episcleral venous plexus and ampulliform dilatations of the conjunctival vessels are usually observed on anterior segment examination. Choroidal hemangiomas occur in up to 50 percent of patients with SWS and may be localized or diffuse. They may cause vision loss from choroidal thickening or retinal detachment. Iris heterochromia, with hyperpigmentation on the affected side of the face, may be noted. Glaucoma is the principal, therapeutically challenging ocular abnormality of SWS.
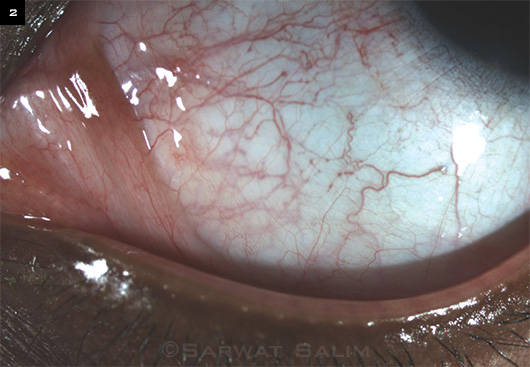 |
|
VASCULAR ANOMALIES. Dense episcleral venous plexus and ampulliform dilatations of the conjunctival vessels on slit-lamp examination.
|
Glaucoma
Glaucoma occurs in 30 to 70 percent of individuals with SWS.3 The incidence of glaucoma increases with PWS involvement of the ipsilateral eyelid and with more severe vascular anomalies of the episclera and conjunctiva. Glaucoma is usually unilateral and often diagnosed in infancy, but it can develop later, during adolescence or adulthood in 40 percent of cases. Bilateral glaucoma can occur in the presence of bilateral facial hemangiomas.
Mechanisms of glaucoma. The two main theories regarding the mechanism of glaucoma in eyes with SWS are developmental anomaly of the anterior chamber angle and elevated episcleral venous pressure, each of which leads to aqueous outflow obstruction. In infancy and childhood, the clinical and histopathological features of the drainage angle in SWS are similar to those seen in primary congenital glaucoma.
On gonioscopy, the angle structures appear indistinct, with a high iris insertion. An anteriorly displaced iris root, poorly developed scleral spur, and thickened uveal meshwork have been observed on histopathological specimens. SWS patients with early-onset glaucoma present with typical signs of congenital glaucoma, including buphthalmos, anisometropia, or amblyopia.
Literature review. Weiss proposed that both congenital angle abnormality and elevated episcleral venous pressure can be responsible for early-onset glaucoma, whereas elevated episcleral venous pressure is the main mechanism underlying late-onset glaucoma.4 In examining 16 eyes with SWS glaucoma, Phelps found episcleral hemangiomas and elevated episcleral venous pressure in all of them; and he postulated that elevated episcleral pressure was the most common glaucoma mechanism in this disorder.5 Cibis and colleagues reported aging changes in the trabecular beams, similar to those seen in primary open-angle glaucoma, in patients with late-onset juvenile SWS.6 A less common mechanism of glaucoma in eyes with SWS is secondary angle-closure glaucoma from retinal detachment or rubeosis.
Management
Glaucoma associated with SWS is generally more difficult to manage than other forms of glaucoma, with a lower success rate and an increased risk of surgical complications. The prominent role played by increased episcleral venous pressure may be responsible.
Early-onset glaucoma. For early-onset glaucoma with associated angle abnormalities, surgical intervention is usually required, with either goniotomy or trabeculotomy. In SWS glaucoma, the success rate of angle surgery is generally lower than in primary congenital glaucoma and often requires additional surgery with trabeculectomy or a glaucoma drainage device. Olsen and colleagues reported intraocular pressure (IOP) of 22 mmHg or less in 66.7 percent of SWS eyes with a median follow-up of 5.4 years after one or more goniotomy or trabeculotomy procedures.7 Iwach and colleagues reported the median stable interval of IOP control after a single goniotomy or trabeculotomy to be 8 and 21 months, respectively, in patients younger than four years.8 Mandal recommended combined trabeculotomy-trabeculectomy in patients with early-onset glaucoma to address both of the underlying mechanisms and reported IOP of 16 mmHg or less without medications over a mean follow-up of 28 months in 10 eyes.9
Late-onset glaucoma. Initial management of late-onset glaucoma is with topical medications; aqueous suppressants and miotics tend to be the most successful. Prostaglandin analogues have been reported to have inconsistent response rates. Laser trabeculoplasty appears to be of limited value.
Trabeculectomy. If topical medication does not produce an adequate response, glaucoma filtration surgery remains the procedure of choice for late-onset cases because it bypasses the episcleral venous system. However, the success of this procedure has been variable in children. Trabeculectomy without antimetabolites is less effective in younger children because of their thicker Tenon’s capsule and more exuberant healing response. Outcomes may be improved with antimetabolites, but these agents increase the long-term risk of blebitis and bleb infection. In addition, trabeculectomy is associated with a much higher risk of choroidal effusions or suprachoroidal hemorrhage in eyes with SWS.
Drainage implant valves. Glaucoma drainage implants have been shown to be effective in eyes with SWS. Hamush and colleagues reported a cumulative probability of success of 79 percent and 30 percent at 24 and 60 months, respectively, in 11 SWS eyes treated with an Ahmed glaucoma valve implant. In this study, success was defined as IOP of less than 21 mmHg without additional glaucoma surgery, suprachoroidal hemorrhage, or retinal detachment.10
Budenz and colleagues reported successful outcomes using a two-stage Baerveldt glaucoma implant in 10 eyes with SWS.3 All eyes had IOP of 21 mmHg or less over a mean follow-up of 35 months without additional glaucoma surgery or suprachoroidal hemorrhage. The two-stage technique allows encapsulation to occur around the plate for a few weeks before placement of the tube in the anterior chamber. This approach minimizes the risk of hypotony and subsequent choroidal effusion or hemorrhage.
Other techniques. Nonpenetrating surgery has also been tried in eyes with SWS glaucoma in an effort to reduce sudden intraoperative and postoperative hypotony.11 Finally, a cyclodestructive procedure may be performed in eyes with failed medical and surgical interventions.
Special considerations for surgery in SWS. In general, lower rates of surgical success and more intraoperative and postoperative complications are observed in patients with SWS-associated glaucoma than in patients with other types of glaucomas. Several precautionary steps can be taken to minimize these complications. These include preoperative reduction of IOP with hyperosmotic agents, placement of posterior sclerotomies prior to entering the eye, preplacement of flap sutures or additional tight sutures during trabeculectomy, use of valved or two-stage glaucoma drainage devices, or radiotherapy of choroidal hemangiomas prior to intraocular surgery.
Klippel-Trénaunay-Weber Syndrome
This syndrome, a rare cause of secondary glaucoma that should be differentiated from SWS, is characterized by a localized or diffuse capillary malformation that overlies a venous malformation and/or lymphatic malformation with associated soft-tissue and bone hypertrophy. The etiology of glaucoma in this syndrome remains unclear, and anomalous angle development and/or elevated episceral venous pressure may be the underlying mechanisms, as has been postulated for SWS.
In addition to the extensive cutaneous hemangiomas affecting the face, similar to SWS, the trunk and limbs are involved. Dysplastic veins and hypertrophy of bone and soft tissues are often present in the affected extremity, which is a lower limb in 90 percent of patients. Malformations of deep veins increase the risk of deep vein thrombosis and pulmonary embolism. Visceral organ involvement is associated with greater morbidity secondary to internal hemorrhage. Cerebral hemangiomas may also occur, causing epilepsy and mental retardation. |
Key Points
SWS is a rare, sporadic disorder involving vasculature in the facial skin, CNS, and eye. The chief ocular manifestation is glaucoma, in which developmental anomaly of the anterior chamber angle and elevated episcleral venous pressure are underlying mechanisms. Because multiple organ systems are affected, SWS is best managed by a multidisciplinary team.
___________________________
1 Shirley MD et al. N Engl J Med. 2013;368(21):1971-1979.
2 Comi AM. Neurologist. 2011;17(4):179-184.
3 Budenz DL et al. Ophthalmology. 2000;107(11):2105-2110.
4 Weiss DI. Trans Ophthalmol Soc U K. 1973;93(0):477-493.
5 Phelps CD. Ophthalmology. 1978;85(3):276-286.
6 Cibis GW et al. Ophthalmology. 1984;91(9):1061-1071.
7 Olsen KE et al. J AAPOS. 1998;2(6):365-368.
8 Iwach AG et al. Ophthalmology. 1990;97(7):904-909.
9 Mandal AK. Ophthalmology. 1999;106(8):1621-1627.
10 Hamush NG et al. Am J Ophthalmol. 1999;128(6):758-760.
11 Rebolleda G. Ophthalmology. 2001;108(12):2152-2153.
___________________________
Ms. Akhter is a graduate student at Harvard, currently studying at Medical College of Wisconsin, and Dr. Salim is professor of ophthalmology and chief of the glaucoma service at Medical College of Wisconsin. The authors report no related financial interests.