Download PDF
Ophthalmologists in the United States now have full access to a prosthetic iris, thanks in part to a decade-long effort spearheaded by the ophthalmic community.
The U.S. Food and Drug Administration gave marketing approval on May 30 to the CustomFlex Artificial Iris for implantation in children and adults with aniridia and other iris defects. Clinical Research Consultants in Cincinnati sponsored the U.S. trial of the device, which is manufactured by HumanOptics.
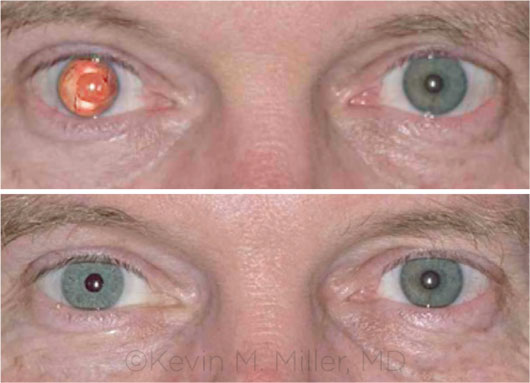 |
BEFORE AND AFTER. This recipient of the artificial iris had aniridia in his right eye. In the bottom image, note the near match of the prosthesis to the color of the fellow eye.
|
New era. The approval marks the end of an era during which U.S. surgeons could implant 1 of the prosthetic irises available overseas only by asking the FDA for a compassionate use exemption. This was a tedious, rule-bound process, and it had to be tackled 1 patient at a time, said Michael E. Snyder, MD, at the University of Cincinnati.
Device details. The CustomFlex implant is a foldable, surface-textured silicone prosthesis that is custom-colored for individual patients, to match the fellow eye. It measures 12.80 mm in diameter, with a pupil diameter of 3.35 mm, and can be injected through an unenlarged 2.2-mm phaco incision. It can be implanted in the capsular bag or sutured to the sclera.
Availability. The device is expected to be commercially available later this year to U.S. surgeons who undergo special training, said Barbara S. Fant, PharmD, president of Clinical Research Consultants, the Cincinnati consulting firm that sponsored the 389-subject trial that led to the device’s approval.
Supporting research. Dr. Snyder and Dr. Fant said they began gathering clinicians’ support for a trial, as well as lobbying HumanOptics to enter the U.S. market, more than a decade ago. The 12-site study began in 2013, with Dr. Snyder and R. Doyle Stulting, MD, PhD, of Atlanta, as medical monitors. Data submitted to the FDA1 included the following:
- Satisfaction. More than 70% of patients experienced decreases in light sensitivity and glare; 94% were satisfied with the artificial iris’ appearance.
- Adverse events. The rate of adverse events was low and included device movement or dislocation (sometimes necessitating repositioning during surgery); increased intraocular pressure (IOP); iritis; synechiae; and secondary surgery to reposition, remove, or replace the device.
- Surgery-related complications. Also minimal, complications included increased IOP, intraocular blood leakage, cystoid macular edema, secondary surgery, corneal swelling, iritis, and retinal detachment.
FDA process. The FDA designated the CustomFlex a “Breakthrough Device” last December, which put the device on an expedited approval pathway. The premarketing approval (PMA) came after close cooperation between the FDA, Clinical Research Consultants, HumanOptics, and the investigators. “To the best of our knowledge, we’re the first ophthalmic device to receive a PMA approval through this new FDA pathway,” Dr. Fant said.
Dr. Snyder noted that the FDA “has been part of this process from day 1. We started off with a very collaborative process with the FDA, and we’ve followed that all the way through.” He added that he is particularly excited about the approval because “patients who previously had no access to this technology are now going to be able to access it within their own community, or in a neighboring community.”
—Linda Roach
___________________________
1 www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm609291.
___________________________
For a video on implanting the artificial iris, see aao.org/clinical-video/using-humanoptics-custom-artificial-iris.
___________________________
Relevant financial disclosures—Dr. Fant: Clinical Research Consultants: E,O; HumanOptics: C; VEO Ophthalmics: E,O. Dr. Snyder: HumanOptics: C,P; VEO Ophthalmics: O.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Connon Atelerix: O.
Dr. Demirci Castle Biosciences: C.
Dr. Fant Alcon: C; BSI: C; Clinical Research Consultants: E,O; CorNeat Vision: C; EyeYon Medical: C; HumanOptics: C; Oasis Medical: C; OptoQuest: C; PromiSight: E,O; Rashmivu: C; Reichert/Ametek: C; University of Louisville Coulter Foundation: C; University of Michigan Coulter Foundation: C; VEO Ophthalmics: E,O.
Dr. Greenberg None.
Dr. Snyder Alcon: S; Bausch + Lomb: S; Glaukos: S; Haag-Streit: C; HumanOptics: C,P; VEO Ophthalmics: O; W.L. Gore: C.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|
More from this month’s News in Review