By Arthur Stone, Contributing Writer, interviewing Zohar Habot-Wilner, MD; Abdulaziz Joury, MD; Ramana S. Moorthy, MD; Priya Samalia, MBChB, PhD; and Gaurav K. Shah, MD
Download PDF
As your patients age, the likelihood that they are taking multiple medications increases. In turn, this raises their risk of drug-induced ocular side effects. Here’s an overview of several selected drugs that are often prescribed to older adults and should be on your differential.
Antiarrhythmics
Amiodarone is used to treat and prevent life-threatening arrhythmias, including atrial fibrillation and ventricular tachycardia (VT).
Impact on the eye. Gaurav K. Shah, MD, in practice in St. Louis, Missouri, noted that “Amiodarone typically causes microdeposits in the cornea, but the verticillata are usually not visually significant.” However, he noted, 1% to 2% of patients can experience optic neuropathy.
In a systematic review of 25 case reports of amiodarone-induced keratopathy published last year,1 coauthors Mona Alshehri, MD, and Abdulaziz Joury, MD, found that the most common findings were cornea verticillata/vortex keratopathy (n = 19; 76%). Fifteen of the patients (60%) saw halos around lights and/or a decrease in vision, and four (16%) reported blurred vision. The median time from initiation of amiodarone therapy to developing ocular side effects was 11.5 months (range, 2-72 months).1
Dr. Joury, a cardiology fellow in New Orleans, said, “We routinely screen patients on amiodarone therapy, especially those patients on high doses as indicated by VT. We start with taking a good history—and ask if the patient has noticed any change in her/his visual acuity, presence of halos around lights, or glare and decreased vision. If so, this leads to further investigation,” including referral to ophthalmology for a slit-lamp examination.
Should therapy be stopped? Earlier research suggests that amiodarone-related toxic optic neuropathy is not a significant concern.2 However, given the potential for visual loss, even if it is infrequent, is there a need to stop amiodarone in all cases of cornea verticillata? “I think the answer is not clear cut, as [the condition] is typically not vision threatening,” Dr. Shah said. “I leave it up to the cardiologist, but I do not recommend stopping it due to corneal issues unless there is a serious adverse effect.”
From the cardiology perspective, the decision to switch medications “depends on multiple factors,” including the underlying cardiac condition, Dr. Joury said. “If the amiodarone can be replaced with an alternative medication with minimal or low ocular adverse effects, it is acceptable to reach out to a cardiologist and inquire about changing medications.”
However, he added, if the amiodarone was prescribed for VT, “unfortunately, the patient has to continue taking it, given the limited number of other antiarrhythmic medications that can prevent future events.” As an alternative, the patient could be referred for VT ablation, Dr. Joury said. “This might decrease the need for long-term treatment with amiodarone.”
 |
|
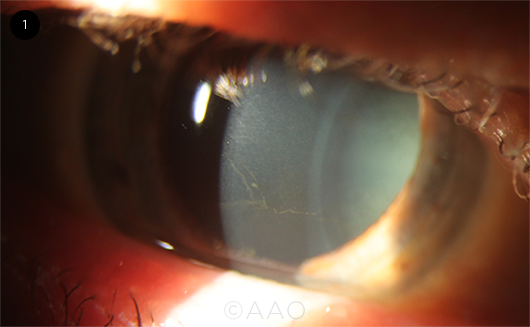
VORTEX KERATOPATHY. Fine, grayish-brown whorl-like opacity branching out across the basal layer of the corneal epithelium, detected in a patient on long-term amiodarone therapy.
|
Bone-Building Medications
Aminobisphosphonates (commonly called bisphosphonates) include alendronate and risedronate, which are in pill form, and pamidronate and zoledronate, which are delivered intravenously. While the drugs in this class are typically prescribed for the treatment of osteoporosis, they also are prescribed as an adjuvant bone-building therapy to patients with metastatic cancer.
Impact on the eye. A number of studies have linked aminobisphosphonates to ocular side effects. As Ramana S. Moorthy, MD, and his coauthors wrote, “Nearly all medications within this class are definitely associated with uveitis, scleritis, episcleritis, and orbital inflammation. Uveitis occurs more commonly in patients without cancer who are being treated primarily for osteoporosis. Intravenous pamidronate sodium is the bisphosphonate most commonly associated with ocular inflammatory disease. There are strong and reproducible data on dose dependent severity of ocular inflammation, de-challenge, and re-challenge (even with different bisphosphonates).”3
Israel. In a retrospective study of patients with orbital inflammatory disease, Israeli researchers found aminobisphosphonate-associated ocular inflammation in eight patients. The median time from drug administration to ocular inflammation was five days (range, 0-14). Aminobisphosphonate therapy was halted in all eight, and some patients received anti-inflammatory treatment.4
“Ocular ultrasound and ultrasound biomicroscopy [UBM] were used in all patients and demonstrated the presence of anterior and posterior scleritis, and myositis,” said Zohar Habot-Wilner, MD, at the Tel Aviv Medical Center.
As part of their study, Dr. Habot-Wilner and her colleagues conducted a literature review to differentiate disease presentation and course between various aminobisphosphonates.3 They found that zoledronate and pamidronate were most likely to cause uveitis. Resolution was achieved in all patients after 1 to 60 days from presentation.
New Zealand. In a retrospective chart review of a uveitis database in New Zealand, drug-induced inflammation occurred in 35 of 2,750 uveitis patients.5 Aminobisphosphonates were the leading culprit (n = 24; 68.6%), followed by etanercept (n = 3; 8.6%) and the B-raf inhibitors vemurafenib and dabrafenib (n = 3; 8.6%). Of the 24 patients who had been on aminobisphosphonates, 22 (91.7%) had anterior uveitis, and two (8.3%) had scleritis.
Lead author Priya Samalia, MBChB, PhD, in practice in Auckland, urged clinicians to take a drug history from any individual presenting with ocular inflammation. “Recurrent ocular inflammation can be vision-threatening.”
Should therapy be stopped? “With bisphosphonates, if patients experience scleritis and uveitis, the physician will need to discontinue the drug,” Dr. Shah said. “Switching to other agents within the same class does not typically work, and patients may need topical steroids to resolve the issues.”
“Another approach could be to pretreat individuals with topical steroids prior to bisphosphonate infusions,” said Dr. Samalia. However, she noted, “This strategy would require further study to see if the risk of uveitis recurrence is reduced.”
Special Considerations: The Cancer Connection
Medication-induced uveitis has become a particular concern with the advent of the class of medication known as immune checkpoint inhibitors (ICIs) as well as with those aminobisphosphonates prescribed to patients with cancer. Treatment of ocular inflammation in these patients requires close collaboration with the treating oncologist. (For a review of ICIs, see “New Cancer Tx, New Ocular Side Effects,” in the July 2020 EyeNet.)
Case study with an aminobisphosphonate. Dr. Habot-Wilner offered the case study of a patient who experienced side effects after receiving zoledronate: “A 58-year-old female, with a medical history of thyroid carcinoma with lung metastasis, was referred to our clinic due to pain, restricted ocular movements, eyelid edema, and redness involving her left eye.” The patient’s complaints started several hours following her first infusion of zoledronate.
Upon examination, the patient’s visual acuity was 20/20 in her right eye and 20/28 in her left, and her IOP was normal in both eyes. Examination of the left eye revealed proptosis, restriction of horizontal ocular movements, ptosis, swelling and erythema of both upper and lower eyelids, severe conjunctival chemosis, and hyperemia. In addition, the patient had mild anterior uveitis with +0.5 cells and no flare in the affected eye. The posterior segment was normal.
“The patient was admitted in order to continue with imaging and blood tests,” Dr. Habot-Wilner said. “UBM ruled out anterior scleral involvement. Ultrasound B-mode demonstrated thickened posterior coats and a T-sign appearance compatible with posterior scleritis.” A CT scan of the patient’s orbit showed proptosis, marked soft tissue enhancement, orbital fat haziness, and scleral thickening compatible with posterior scleritis and diffuse orbital inflammation.
With regard to blood tests, “C-reactive protein was high at 61 mg/L (normal, 0-5 mg/L), but there were no other abnormal laboratory findings suggestive for diseases related to ocular inflammation, and the patient was not taking any other medications that could cause the inflammatory eye disease,” Dr. Habot-Wilner said. The patient was diagnosed with mild anterior uveitis and orbital inflammation secondary to the use of zoledronate, and the medication was discontinued.
“Several hours after her admission to our ward, she reported that her pain had lessened, and our examination revealed improvement of the swelling and hyperemia,” Dr. Habot-Wilner said. Three days later, the patient had markedly improved with complete resolution of her pain, proptosis, periocular edema, and conjunctival chemosis. Two weeks after her initial presentation, the only ocular sign was a very mild conjunctival hyperemia—and a week later, all examination findings were normal.
Case study with an ICI. Dr. Moorthy offered the following case study: A 58-year-old woman who was being treated for refractory metastatic breast cancer was started on ipilimumab, an ICI often used to treat melanoma. After the second month of therapy, she presented with pain, photophobia, and moderate vision loss in her right eye.
Examination revealed inflammatory cells in the anterior chamber and vitreous of her right eye, with unilateral multifocal choroidal lesions in the periphery and cystoid macular edema (CME). It was thought that she had ipilimumab-induced uveitis.
“Because of the patient’s immune-compromised health status, we ordered tests for her panuveitis, including QuantiFERON-TB, antitreponemal IgG, serum angiotensin-converting enzyme level, serum lysozyme level, and chest radiograph. All were negative or normal,” said Dr. Moorthy, in practice in Indianapolis.
Dr. Moorthy treated the patient with periocular corticosteroid therapy, topical corticosteroids, and cycloplegics, and her inflammation and CME resolved. Because the patient experienced initial reduction of tumor burden, the ipilimumab was continued by the oncologist while local corticosteroid therapy was maintained for several months. Unfortunately, due to tumor recurrence, treatment was eventually discontinued, and the patient was placed in hospice care, Dr. Moorthy said.
__________________________
1 Alshehri M, Joury A. Optom Vis Sci. 2020;97(7):536-542.
2 Mindel JS et al. Am Heart J. 2007;153(5):837-842.
3 Moorthy RS et al. Curr Opin Ophthalmol. 2018;29(6):588-603.
4 Keren S et al. Acta Ophthalmol. 2019;97(5);e792-e799.
5 Samalia P et al. N Z Med J. 2020;133(1527):83-94.
__________________________
Dr. Habot-Wilner is clinical associate professor of ophthalmology at the Sackler Faculty of Medicine at Tel Aviv University as well as director of the uveitis and inflammatory disease service and a retina consultant at the Tel Aviv Medical Center, both in Tel Aviv, Israel. Relevant financial disclosures: None.
Dr. Joury is a cardiology fellow at Ochsner Health in New Orleans. Relevant financial disclosures: None.
Dr. Moorthy is clinical associate professor of ophthalmology at Indiana University’s Eugene and Marilyn Glick Eye Institute and in practice with Associated Vitreoretinal and Uveitis Consultants, both in Indianapolis. Relevant financial disclosures: None.
Dr. Samalia is a uveitis fellow at the Greenlane Clinical Centre in Auckland, New Zealand. Relevant financial disclosures: None.
Dr. Shah is a partner at The Retina Institute in St. Louis, Mo. Relevant financial disclosures: None.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Habot-Wilner AbbVie: C,L; Novartis: C.
Dr. Joury None.
Dr. Moorthy None.
Dr. Samalia None.
Dr. Shah Allergan: C,L,S; DORC: C; Novartis: C; OMIC: C; Regeneron: C,L,S.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|