Download PDF
NEI scientists have successfully demonstrated a method for converting human blood progenitor cells into stem cells that, in turn, differentiate into retinal pigment epithelial (RPE) cells capable of keeping photoreceptors healthy.1
The ultimate goal is to protect at-risk photoreceptors by transplanting patient-specific sheets of functioning RPE cells into eyes with dry age-related macular degeneration (AMD), said principal investigator Kapil Bharti, PhD, at the NEI.
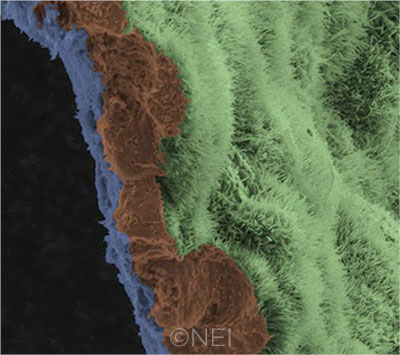 |
IN THE LAB. A scanning electron micrograph image shows a polarized RPE monolayer on a biodegradable scaffold. The image is colored to highlight the scaffold (blue), three RPE cells (brown), and the apical process of cells in the RPE monolayer (light green).
|
Animal model. The researchers described a painstaking process for transforming progenitor cells into induced pluripotent stem cells (iPSCs), then inducing differentiation into RPE cells, which are grown in a monolayer atop a biodegradable scaffold.
“We start with the patient’s own blood, isolate the blood progenitor cells, and reprogram them into induced pluripotent stem cells—that is, cells that can make any type of cell in the body,” Dr. Bharti said. “And you can imagine the advantage, then, if you make a tissue out of these cells: It becomes the patient’s own tissue, so the immune system would not reject it.”
Using a specially designed transplant delivery cannula, the researchers inserted single-source sheets of cells between the photoreceptors and the dying RPE layer in rodents and pigs. The patch size was 2 mm × 4 mm in the pigs, the same size as a human clinical dose would be, Dr. Bharti said.
Functional results. Imaging, molecular, and electrical studies during up to nine months of follow-up found that the patches of transplanted cells functioned well and without evidence of toxicity. The laboratory-grown RPE cells integrated appropriately into the animals’ retinas as the biopolymer scaffold degraded. They also expressed RPE65 (the gene that drives regeneration of the ocular photopigment rhodopsin), performed the RPE’s crucial function of pruning photoreceptors through phagocytosis, and facilitated normal electrical responses from the rescued photoreceptors adjacent to the implanted cells.
Because of concerns about possible oncogenic potential in tissue derived from stem cells, the researchers also performed genetic analyses of the iPSCs and found no mutations that are known to be associated with tumor growth, they reported.
Planning for clinical trial. Dr. Bharti said the group’s cellular production processes strictly followed “good manufacturing practice” protocols, in order to facilitate FDA approval of an early clinical trial, which the researchers hope will begin this year. Planning is underway for a phase 1/2a trial in patients with geographic atrophy and visual acuity of no better than 20/200, he said.
If this and further clinical studies were to demonstrate safety and efficacy, transplants of this lab-grown RPE tissue could be submitted to the FDA for commercial approval in three to five years, Dr. Bharti estimated.
Dr. Bharti said the researchers are cautiously optimistic about the potential that this individualized approach eventually could have for AMD patients with geographic atrophy. “If implanted in the right place, [these cells] would stop the disease from progressing further—and this is in a disease where there currently is no treatment available.”
—Linda Roach
___________________________
1 Sharma R et al. Sci Transl Med. 2019;11(475):eaat5580.
___________________________
Relevant financial disclosures—Dr. Bharti: None.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Bharti None.
Dr. Koch Alcon: C; Carl Zeiss Meditec: C; Johnson & Johnson: C.
Dr. Nouri-Mahdavi Heidelberg Engineering: L,S.
Dr. Pointdujour-Lim None.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|
More from this month’s News in Review