Download PDF
In last month's Clinical Update, four experts described the reasons for and role of digital retinal photography in screening for retinopathy of prematurity (ROP). In Part 2, these early adopters share the practical lessons they’ve learned in setting up successful ROP telemedicine programs.
Program Planning and Adoption
There is no off-the-shelf model for an effective ROP screening program. However, there are common elements in starting the process: identifying needs and benefits and obtaining buy-in from all stakeholders.
Needs: Overcoming workforce and training challenges. Adding telemedicine to an institution’s screening protocols might help overcome workforce and training difficulties, according to Michael F. Chiang, MD, at Oregon Health & Science University in Portland. A recent Web-based survey he and his colleagues conducted of pediatric ophthalmology and retina fellows’ training for ROP care raised some concerns.1
“In a lot of cases, our trainees are not learning this very well and are sometimes missing critical diagnoses,” he said. “And in many major institutions, fellows are the only ones doing the ROP exams—no attendings are seeing these babies.” One-third of survey respondents said they were the only ones examining babies in up to two-thirds of cases.
“This gets to the fact that it’s hard to examine babies, tough to get to the newborn intensive care unit [NICU], and challenging to get two people there at the same time,” he said. “Sometimes the ophthalmologists doing the exams may not want to do them and may not be the most experienced at doing them.”
Benefits: Improving efficiencies. When you’re considering a telemedicine program, try to quantify how it might facilitate your practice, advised Graham E. Quinn, MD, MSCE, at the Children’s Hospital of Philadelphia. Dr. Quinn is the lead investigator for the multicenter e-ROP trial.
Are you going to the nursery every week to perform binocular indirect ophthalmoscopy (BIO) on babies? Could screening via telemedicine, even in your own hospital, help you extend that period to every two, or even four weeks—only when babies must be examined to consider treatment? Dr. Quinn said that it could be a real timesaver if telemedicine could reduce by 20 to 50 percent the number of babies who need in-person ROP screening. These potential efficiencies might serve as an opening to conversations with hospital administrators about support for a telemedicine program.
Getting broad buy-in. Darius M. Moshfeghi, MD, is director of telemedicine at Byers Eye Institute at Stanford University Medical Center in Palo Alto. He founded and directs the Stanford University Network for Diagnosis of Retinopathy of Prematurity (SUNDROP) telemedicine screening initiative, which has reported 100 percent sensitivity for the detection of treatment-warranted ROP.2
His advice? Think broadly and get buy-in from everyone involved at all institutions involved. This includes nursing and NICU staff, administrators, and malpractice attorneys. “There are a lot of stakeholders in these contracts, and you should never provide screening before every possible outcome has been stipulated,” he said. (See sidebar, “Critical Questions.”)
In addition, said Daniel T. Weaver, MD, pediatric ophthalmologist in Billings, Mont., buy-in includes involvement of information technology departments. “You have to have a reliable, secure Internet connection that is HIPAA compliant,” he said.
|
ROP Progression
|
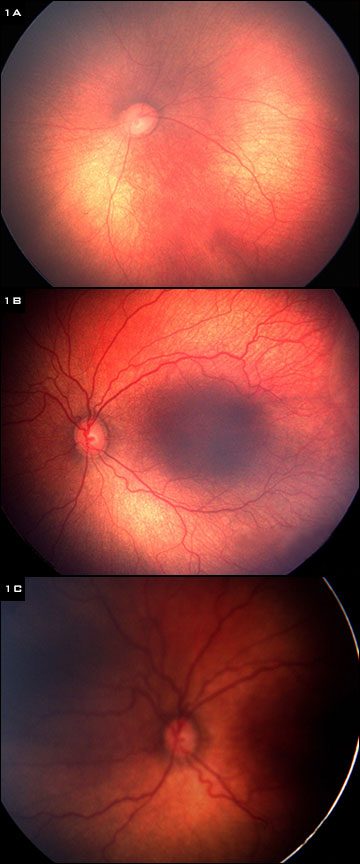 |
|
(1) Fundus of infant born at 24 weeks, weighing 700 g. (A) Normal exam at 31 weeks gestational age. (B) Exam at 34 weeks shows borderline pre-plus disease. (C) Exam at 37 weeks shows progression to plus disease.
|
Implementation
24/7 commitment. The foundation of a successful program is daily commitment from both doctors and imagers, said Dr. Weaver. “You really have to stay in touch with the unit to make sure there’s coverage if you’re going to be unavailable,” he said. “You can’t let even one baby fall through the cracks.”
Consider the imager. What are the characteristics of a good imager? “It should be someone who is familiar with handling tiny babies, sensitive to what’s going on, and quick to respond if the heart rate slows down or the baby stops breathing,” said Dr. Quinn. Neonatal nurses are particularly good, said Dr. Weaver, because they’re closely attuned to neonates, but neonatologists are also excellent choices. “I think anyone with a light touch can be trained to use the camera to examine these babies.”
Training. Dr. Moshfeghi recommends a mentoring program for anyone who intends to do ROP screening—whether by camera or in person with BIO. “If you are not capable of or not willing to do that, you shouldn’t be screening,” he said. By the same token, he added, patience is pivotal for the mentoring ophthalmologist because many of these imagers have never taken photographs of the human eye, let alone of a premature baby’s eye. “You have to show your photographers ‘a lot of love,’ encouraging them in the early days and providing additional hands-on training after they have been screening for several weeks.”
Consistency is key. “Part of the reason we’ve been successful,” said Dr. Weaver, “is that we’ve had the same two screeners since 2007. These nurses do this so often they’ve become expert imagers. Not only are they good at imaging, but they also recognize ROP. If they see a baby they’re worried about, they send the images, but they also call us and say, for example, ‘You really need to look at image 19 of the left eye on Baby Smith.’”
Referral criteria. Dr. Weaver (and Missoula pediatric ophthalmologist Todd Murdock, MD) offers ROP telemedicine screening for a Great Falls, Mont., NICU that lost its coverage for screening. Dr. Weaver said that the referral criteria were purposely set low to avoid missing any baby with treatment-warranted ROP. In his program, infants are transported to an appropriate facility for in-person exam if any of the following are present on digital imaging: 1) type 2 or worse ROP; 2) any plus disease; 3) zone II stage 2 ROP with pre-plus disease; or 4) eyes that cannot be imaged well.3
The criteria for transport may differ depending upon the region, said Dr. Weaver. In some cases, doctors may be able to drive to an NICU for an onsite exam; but in Montana, severe weather and geography often dictate when transportation is even possible. “If I had a baby I was worried about, I would have to wait for the weather to clear and fly them down by air ambulance,” he said, adding that transportation arrangements were spelled out ahead of time in contracts with the hospital.
Screen regularly. Both Dr. Moshfeghi and Dr. Weaver recommend screening babies weekly, when possible, to observe the rate of change and safeguard against missing progression. “When we published our results, some reviewers felt that weekly screening was excessive,” said Dr. Weaver. “But that is the price I accept. I would rather do a few extra exams than miss even one baby.”
Weekly screenings have been a real eye-opener for Dr. Moshfeghi, who said it’s important to remember that screening criteria have been stipulated based upon findings noted during BIO. The camera images do not routinely allow viewing of zone III; therefore, far peripheral disease may be missed.
“To account for this, I screen weekly,” he said. “When looking at individual images, sometimes I’m not that worried. But then when I look at a series of images and compare them with the original image, I can see that a baby is getting worse. It’s given me a profound appreciation for how much this disease can move.”
A major concern about telemedicine screening, said Dr. Quinn, is missing a baby who needs an exam, but the robust systems described by Drs. Weaver and Moshfeghi minimize this possibility.
Critical Questions
In setting up a new telemedicine program—as in most other complex undertakings—the devil is in the details. Before you proceed, the experts advised resolving the following questions, some of which have already been addressed in traditional ophthalmoscopic screening protocols.
How will you
- Train people to take good photos?
- Maintain a level of quality control and certification?
- Get a retake or transfer the baby if the person reading images feels the quality is inadequate?
- Ensure a bulletproof system, in which you can look at the images and return a reading back to the NICU to take further action, if necessary?
Who is responsible for
- Reading photos if they are sent to a reading center?
- Acting as a backup if the imager calls in sick?
- Fixing a machine if it breaks?
- Paying for telemedicine?
- Covering malpractice?
What steps should be taken
- If the baby needs treatment?
- If images are not of good quality?
|
Fine-Tuning for the Future
Many telemedicine hurdles have already been overcome. “For example, it used to be difficult to get images in a HIPAA-compliant e-mail,” said Dr. Moshfeghi. “Similar to iPhone syncing, we now have automatic, secure syncing with our server.” Still, some challenges remain.
A focus on the camera. “We need a camera that’s more robust and cheaper,” said Dr. Quinn. “And it would be wonderful to have one that could be held 3 to 4 inches away from the baby, rather than floating on a gel as it currently does. This would help cut down on the risk of infection.”
Dr. Moshfeghi said that the only camera thus far that has demonstrated long-term efficacy for ROP screening is the Clarity RetCam with a 130-degree lens. “Any new lens or camera with a less angular view will need to be validated in a series of studies over years.”
Cost control. Imagers currently take five retinal images plus an external image to determine pupil size, said Dr. Quinn. He and Duke University pediatrics professor Alex R. Kemper, MD, MPH, MS, are seeking ways to streamline screenings. “Could we look just at the three horizontal images … or just at the posterior pole? And are there ways to computerize some analyses—reading right at the bedside, rather than having to upload to a reading center or to a physician reader?”
ROP telemedicine could become highly cost-effective, he said, pointing to diabetic retinopathy as a model, where a clear scoring system used in the screening process guides the approach to treatment and follow-up. “The timeline is more complicated with ROP, since feedback needs to be very quick, but I think we can come up with a paradigm for these babies within the next four to five years.”
Adding algorithms. Progress has already been made in this direction. A variety of algorithms (WINROP, CHOP ROP, G-ROP) now help identify babies’ level of risk by evaluating their gestational age, birth weight, and rate of weight gain per day.4 Such algorithms, said Dr. Quinn, may help distinguish between the babies who require early, frequent screening and those who can be screened less often.
Expanding the use of the camera. Another path to cost-effectiveness could involve using the camera for other purposes, such as universal screening, said Dr. Moshfeghi. “We’ve decided we don’t want kids to be deaf, offering all newborns hearing testing,” he said, “and we clearly don’t want them to be blind either. At Stanford, we’re offering universal screening to all infants within 72 hours of birth to identify any eye disease as part of the Newborn Eye Screen Testing (NEST) Study, with longitudinal follow-up. We’ve found all kinds of fascinating things we wouldn’t have suspected. And it was easy to implement because we already had the camera—we’ve just increased its duty cycle.”
___________________________
1 Wong RK et al. J AAPOS. 2013;16(2):177-181.
2 Fijalkowski N et al. Current Eye Res. 2013;38(2):283-291.
3 Weaver DT et al. J AAPOS. 2012;16(3):229-233.
4 Lundgren P et al. PLoS One. 2013;8(9):e73256.
___________________________
Michael F. Chiang, MD, is professor of ophthalmology and medical informatics and clinical epidemiology at Oregon Health & Science University, in Portland. Financial disclosure: Is an unpaid member of the scientific advisory board for Clarity Medical Systems, the manufacturer of RetCam.
Darius M. Moshfeghi, MD, is director of telemedicine at Byers Eye Institute and associate professor of ophthalmology at Stanford University Medical Center, in Palo Alto, Calif. Financial disclosure: Has equity in Visunex Medical Systems.
Graham E. Quinn, MD, MCSE, is professor of ophthalmology at Children’s Hospital of Philadelphia. Financial disclosure: Has received NEI support for e-ROP.
Daniel T. Weaver, MD, is a pediatric ophthalmologist in Billings, Mont. Financial disclosure: None.