By Rebecca Taylor, interviewing Brandon D. Ayres, MD, Kevin M. Miller, MD, and Michael E. Snyder, MD
Download PDF
For the past decade, U.S. surgeons have implanted the occasional artificial iris under labyrinthine compassionate use protocols. Following FDA approval of the CustomFlex artificial iris (HumanOptics; distribution by VEO Ophthalmics) in 2018, surgeons now have easier access to the device. And results of a recently published, FDA-sanctioned study found that the prosthesis met or surpassed all key safety and efficacy endpoints.1 (See “Trial Results: Safety, Efficacy, and Cosmesis.”)
However, implantation can be a complex surgical procedure, said Brandon D. Ayres, MD, at Wills Eye Hospital in Philadelphia and lead author of the study. In addition, significant training is required before a clinician can be credentialed as an implanting surgeon.
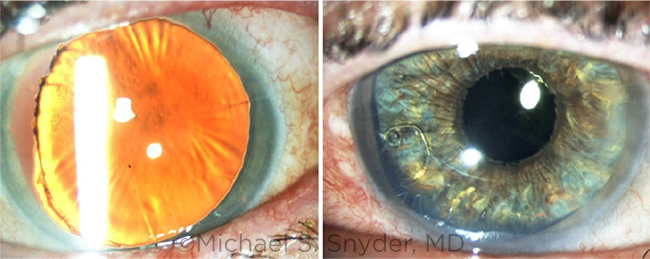 |
|
BEFORE AND AFTER. (Left) Pre-op image shows a mydriatic and atrophic iris with zonular dialysis. (Right) Post-op image shows the custom iris in the capsular bag, fixated by a Cionni modified CTR. The residual iris tissue has darkened a bit from inflammation compared to the fellow eye.
|
Difficult Cases
Congenital or acquired aniridia. Eyes that require an iris prosthesis have congenital or acquired aniridia, and they usually have significant ocular comorbidities, said Michael E. Snyder, MD, at the Cincinnati Eye Institute.
“These are super-sick eyes,” agreed Kevin M. Miller, MD, at the Stein Eye Institute at the University of California, Los Angeles (UCLA). Whether the patient was born without an iris or lost the iris in what he called “a horrific trauma,” they typically have “a mixed bag of cornea problems, glaucoma problems, detached retinas, failed corneas, and missing or dangling lenses.”
With regard to acquired aniridia, “Patients often have iris defects after surgical repair for a ruptured globe,” Dr. Ayres noted. “After the primary repair is completed, they’re often left with significant damage to the anterior segment.”
Symptoms. Light-related symptoms of aniridia include photophobia, glare, arcs, halos, and starbursts. Pseudophakic patients often experience double or shadow images because light is both focused by the IOL and goes around it through the iris defects, thus exposing the aphakic space, Dr. Snyder said.
Patient desperation. Without a working iris, “patients patch their eye, wear dark sunglasses, or get a blackout contact lens,” Dr. Miller said. “There really were no good options” before the CustomFlex became available.
Contraindication. “There’s one absolute contraindication from the FDA: We can’t implant an artificial iris into a phakic eye with a clear lens,” said Dr. Miller. “The artificial iris doesn’t open and close like a normal pupil, so we can’t use dilating drops to do cataract surgery.”
Instead, cataract surgeons must wait until a cataract develops and do lens and iris implant surgeries simultaneously. The mechanics of placing the prosthesis into the anterior segment could induce a cataract, added Dr. Snyder, and one European case report has described this phenomenon.2
In addition, the artificial iris is contraindicated for pregnant women, children under 3 years of age, and patients with several ocular conditions, including proliferative diabetic retinopathy and uncontrolled or severe uveitis.
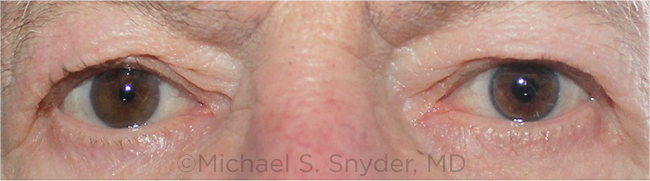 |
|
COLOR MATCH. This midface photo shows the CustomFlex iris in the patient’s left eye with a good color match to the fellow (right) eye.
|
A Custom-Made Iris
The iris has a fixed aperture of 3.35 mm and comes in two models. The “with fiber” model sports an embedded polyester mesh for active suture fixation to residual iris tissue, sclera, or an IOL. The “fiber-free” model is often used for passive fixation in the capsular bag or sulcus space, although it can also be suture fixated, said Dr. Snyder.
“The fiber-free model is pure silicone, very flexible and thin,” said Dr. Ayres. “I typically choose that version if I’m confident I can place the iris in the capsular bag.” In contrast, the with-fiber prosthesis has an embedded meshwork that improves its durability but decreases elasticity. Thus, he often chooses this model if he’ll be suture-fixating the iris to the scleral wall.
The custom-made devices are matched to an image of the fellow eye’s intact iris, a relative’s iris, or a stock image for congenital aniridics, said Dr. Snyder. “I describe it as a really good ‘cocktail party’ match, meaning that, at cocktail party lighting and distance, it looks pretty close to the other eye.”
And eye color matters. “With a lightly colored iris, if the pupil is not dead on, you can see it from across the room,” said Dr. Miller, “but with brown eyes, it can be off, and nobody notices.”
Robust Surgical Training Required
VEO Ophthalmics also oversees access to and training criteria for the device in the United States. They require significant previous experience with complex anterior segment cases, said Dr. Miller. “The iris itself is pretty easy to deal with, but you need the skill set to deal with all of the other problems [typically encountered] with these eyes.”
Surgeons must attest to their threshold of experience (e.g., number of capsular tension rings [CTRs], number of scleral suture-fixated lenses) before a robust online training, said Dr. Snyder. “If they pass, VEO sends them two SimulEye surgical training models: one with an artificial capsular bag, the other aphakic with no capsular structures.”
For dry lab training, surgeons may use their own equipment in their ORs so that they will become comfortable with the surgical maneuvers needed, Dr. Snyder said. “Surgeons who use this device should be comfortable dealing with zonular dialysis cases and putting in CTRs, be able to do anterior vitrectomy with both limbal and pars plana incisions, and have ample experience suture-fixating implant lenses to the scleral wall.”
Surgical Pearls
Essentially, the surgical approach for the artificial iris comes down to the following basic questions, said Dr. Miller: “Where in the eye are you going to put the device? Where—and how—are you going to fixate it? And how are you going to insert the device inside the eye?”
Placement. “You can place the iris inside the capsule, where a lens implant goes, or in front of the capsule in the sulcus space, right behind where the native iris tissue would be,” Dr. Miller said.
Fixation. With an intact capsule, “we always put in a CTR, and then, optionally, we can place the iris inside the capsular bag as well, which passively fixates it,” he said. “Alternatively, if there’s any residual iris tissue, surgeons can drop the device into the sulcus space and passively fixate it there.”
With regard to active fixation, “One method involves suturing the artificial iris to an IOL and then suturing the lens to the sclera,” Dr. Miller said. Alternatively, the surgeon can suture the lens implant to an artificial iris and then suture the iris to the sclera. This achieves “four-point fixation for a little less wobble than the two-point fixation in the first technique,” he said.
Insertion. “We can use an old-style lens injector that accommodates a large lens or insert it with forceps,” Dr. Miller noted. “We also can ‘dunk it’ with almost any instrument if we’re doing a corneal transplant at the same time.”
Expect surgical surprises. Unexpected turns often happen in the OR with these eyes, said Dr. Snyder. For instance, the surgeon might plan to implant the artificial iris in the capsular bag but find the existing support structures unstable. “Then we’d place an implant lens and artificial iris with scleral fixation.” And Dr. Ayres offered the following tips:
Prepare for multiple scenarios. “Have your primary plan and then plan Plan B, Plan C, and even Plan D, just in case,” he said. “You’ve got to respect these eyes.”
Double-check surgical instruments. “These cases require more instrumentation than you would usually have during cataract surgery, so have your tool kit set up,” Dr. Ayres said. He uses an intraocular ruler and a Silver Series inserter (Johnson & Johnson), “and it’s also recommended to have a microanterior segment set, a microforceps, and centration devices.”
Resize the implant. “Err on the side of a bit smaller,” Dr. Ayres recommended. “The prosthesis comes at 12.8 mm edge to edge, so use a round trephine to size the prosthesis properly.”
Bottom Line
Overall, the uplift to patients’ well-being is remarkable, said Dr. Ayres. In reflecting on his participation in the artificial iris study, he said, “Seeing the journeys these patients went through, improving their feelings of self-confidence, and helping them see better—this was the most rewarding study I’ve ever been involved with.”
___________________________
1 Ayres BD et al. Ophthalmology. Published online Feb. 4, 2022.
2 Magnus J et al. J Cataract Refract Surg. 2012;38(6):1097-1100.
___________________________
Dr. Ayres is a member of Ophthalmic Partners in the Philadelphia area and serves on the cornea service at Wills Eye Hospital in Philadelphia. Relevant financial disclosures: None.
Dr. Miller is professor of clinical ophthalmology at the David Geffen School of Medicine at UCLA and chief of the Cataract and Refractive Surgery Division of the Stein Eye Institute in Los Angeles. Relevant financial disclosures: None.
Dr. Snyder is professor of ophthalmology at University of Cincinnati and practices at the Cincinnati Eye Institute. Relevant financial disclosures: HumanOptics C,P; VEO Ophthalmics P,S.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Ayres Alcon: C,L; Allergan: C,L; Bausch + Lomb: L; Carl Zeiss: C; CorneaGen: C; Dompé: C,L; Glaukos: C; New World Medical: C; Novartis (Alcon): C; Omeros: C; Sun Ophthalmics: C,L; Tear Lab: C.
Dr. Miller Alcon: C; Johnson & Johnson Vision: C,S; Lensar: C; Oculus: C.
Dr. Snyder Alcon: S; Alnivo: C; Glaukos: S; Haag-Streit USA: C; HumanOptics: C,P; Johnson & Johnson Vision: S; Trefoil: S; VEO Ophthalmics: P,S; W.L. Gore: C.
.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Hired to work for compensation or received a W2 from a company. |
| Employee, executive role |
EE |
Hired to work in an executive role for compensation or received a W2 from a company. |
| Owner of company |
EO |
Ownership or controlling interest in a company, other than stock. |
| Independent contractor |
I |
Contracted work, including contracted research. |
| Lecture fees/Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Patents/Royalty |
P |
Beneficiary of patents and/or royalties for intellectual property. |
| Equity/Stock/Stock options holder, private corporation |
PS |
Equity ownership, stock and/or stock options in privately owned firms, excluding mutual funds. |
| Grant support |
S |
Grant support or other financial support from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and\or pharmaceutical companies. Research funding should be disclosed by the principal or named investigator even if your institution receives the grant and manages the funds. |
| Equity/Stock/Stock options holder, public corporation |
US |
Equity ownership, stock and/or stock options in publicly traded firms, excluding mutual funds. |
|