By James A. David, MD, and Jared M. Vincent, MD
Edited by Ahmad A. Aref, MD, MBA
Download PDF
Paula Pearl,* a 55-year-old Black woman, rescheduled her diabetic retinopathy fundus photo screening multiple times over a two-year period. At her appointment, she did not express concerns about her vision. Fundus photography of her right eye showed a yellow-orange subfoveal lesion that extended superiorly and a separate lesion of the right temporal macula. The left fundus was normal. We scheduled her for an appointment in the retina clinic for further evaluation.
At the Retina Clinic
Following an additional five-month delay, Ms. Pearl presented to the retina clinic and reported seeing a “worsening brown spot” in her right eye.
History. Ms. Pearl had a past medical history of hypertension, hyperlipidemia, and well-controlled type 2 diabetes without significant previous ocular history. During the previous two years, she had reported to the emergency department (ED) on three occasions after motor vehicle accidents. The ED notes documented all three collisions as “minor,” and there was no evidence of ecchymosis, lacerations, or other head, eyes, ears, nose, and throat (HEENT) abnormalities. None of the accidents involved direct ocular trauma.
Vision and anterior segment exam. Uncorrected visual acuity (VA) in the right eye had deteriorated from 20/80 at the earlier fundus photo screening to counting fingers (CF), but the left eye was stable at 20/50. Both eyes had normal IOP. Pupils were round and reactive without afferent pupillary defect, extraocular movements were full, and confrontation fields were full to counting fingers. Both eyes had a mild nuclear sclerotic cataract.
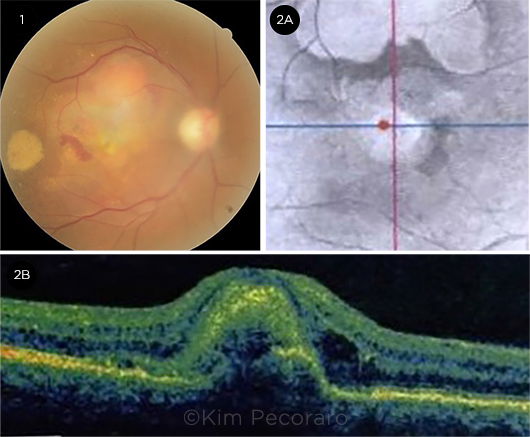
Funduscopic exam. Funduscopic exam of the right eye revealed a sub-foveal yellow-orange retinal lesion that was 2 to 3 disc diameters in size. There was superior elevation, as well as superonasal and temporal subretinal hemorrhage (Fig. 1).
The left eye fundus examination was normal.
OCT. OCT near-infrared images (Fig. 2A) of the right eye showed central and superior abnormalities. B-scan cross sections (Fig. 2B) showed a subretinal complex with intraretinal and subretinal fluid consistent with choroidal neovascularization. The subretinal complex at the fovea was surrounded by subretinal fluid nasally, temporally, and superiorly.
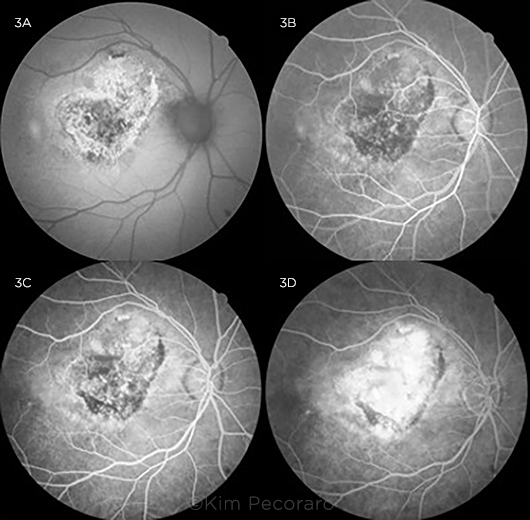
Other imaging. Fundus autofluorescence (FAF) imaging of the right eye taken at subsequent visits showed stippled central hyper- and hypoautofluorescence surrounded by a sharply demarcated ring of hyperautofluorescence (Fig. 3A). Fluorescein angiography (FA) of the right eye demonstrated early hypofluorescence centrally, consistent with blocking, followed by late hyperfluorescence consistent with staining and leakage (Figs. 3B-3D).
 |
|
WHAT WE SAW AT THE RETINA CLINIC. (1) A fundus photo shows a yellow-orange subfoveal lesion with nasal subretinal hemorrhage, a temporal subretinal hemorrhage with associated exudation, and retinal thickening superiorly. (2A) An OCT near infrared image shows variable reflectivity. (2B) An OCT B-scan section through the fovea demonstrates subretinal elevation centrally with distortion of foveal contour and adjacent intraretinal hyporeflectivity consistent with intraretinal fluid.
|
Initial Misdiagnosis
We made a diagnosis of choroidal neovascular membrane (CNVM) and presumed its origin to be idiopathic due to negative history of infections or inflammatory disorders, absence of intraocular inflammation, and lack of direct trauma.
Ms. Pearl received an intravitreal injection of bevacizumab and was followed monthly. After six injections, the fluid continued to resolve, and her VA improved to 20/200. But she started to miss appointments, and VA in her right eye subsequently declined to CF. Change of therapy to aflibercept improved her vision to 20/100.
However, with poor follow-up and return of subretinal fluid, Ms. Pearl’s vision subsequently regressed to CF despite multiple aflibercept and ranibizumab injections.
 |
|
FUNDUS AUTOFLUORESCENCE AND FLUORESCEIN ANGIOGRAM OF THE RIGHT EYE. (3A) FAF shows stippled central hypoautofluorescence surrounded by a sharply demarcated ring of hyperautofluorescence. (3B) FA shows decreased central fluorescence consistent with blocking in arteriovenous phase, (3C) increased fluorescence consistent with staining versus early leakage during the venous phase, and (3D) significantly increased central hyperfluorescence with slightly indistinct margins most consistent with leakage in the recirculation phase.
|
No Longer “Idiopathic”
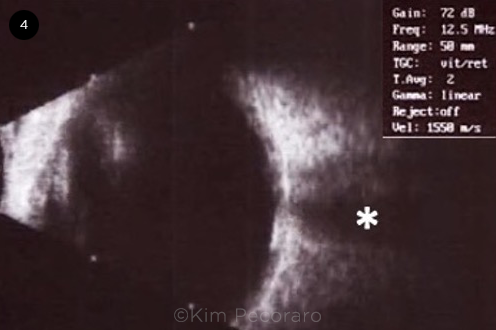
Three years after the initial diagnosis of idiopathic CNVM, a different physician examined the patient and performed B-scan ultrasonography, which demonstrated that the lesion was hyperechogenic with a high A-spike and shadowing consistent with choroidal calcification (Fig. 4). The diagnosis was changed to choroidal neovascularization secondary to choroidal osteoma.
 |
|
ULTRASOUND B-SCAN OF THE RIGHT EYE. B-scan ultrasound of the central macula shows hyperreflectivity at the level of the choroid with posterior acoustic shadowing (asterisk).
|
A Rare Finding
Choroidal osteomas are composed of ectopic mature bone tissue that grows in the choroid, most often in a peripapillary location. The first known case was presented at a meeting of the Verhoeff Society in 1975, and Gass et al. published the first case series of four patients in 1978.1
When we searched PubMed, we found that 135 cases were reported between 2010 and 2017. Shields et al. recorded 74 eyes of 61 patients with choroidal osteoma across 26 years, and Helsinki University Hospital estimated an incidence of 1 in 5 million.2
Roughly 80% of cases are unilateral. There is a female predominance with a female:male ratio of 2:1. The condition is usually detected in early adulthood, but pediatric cases have been documented in patients as young as 3 years old.1
Differential Diagnosis
The differential diagnosis for choroidal osteomas includes other types of ocular calcifications and tumors, such as sclerochoroidal calcification, choroidal hemangioma, choroidal melanoma, choroidal carcinoma, choroidal nevus, organoid nevus syndrome, posterior scleritis, and posterior scleral choristoma.
Sclerochoroidal calcification versus choroidal osteoma. Sclerochoroidal calcification occurs bilaterally in roughly half of cases and, compared with choroidal osteoma, targets an older demographic (mean age, 69 years old versus 28 years), is more likely to be multifocal, and appears along the vascular arcades as compared to a posterior or peripapillary location in osteomas.3
Pathogenesis
The exact origin of choroidal osteomas is unknown. Theories have included a metaplastic response to inflammation, trauma, or hormone levels, while some researchers have reported a genetic predisposition. However, the most recent hypothesis proposes that choroidal osteomas are congenital choristomas. Following years of ossification, the bone tissue eventually deossifies, which damages the outer retina, photoreceptors, and retinal pigment epithelium (RPE). A sight-threatening CNVM forms in 31% to 47% of cases.3 This may be followed by accumulation of subretinal fluid or hemorrhage.
Imaging Characteristics
Choroidal osteomas can remain asymptomatic for years and usually are not diagnosed until visual impairment has developed. Although multiple imaging modalities have been used in the diagnosis and characterization of these tumors, funduscopy and B-scan ultrasonography are sufficient for diagnosis. The classic funduscopic appearance is a white-cream, yellow-gray, or orange lesion with well-defined, scalloped margins frequently located in the peripapillary region. The orange appearance may be more prevalent in areas with more ossification.4 Alternatively, a yellow hue may be due to RPE degeneration.3 One series found basal diameter to range from 3 to 20 mm and thickness to range from roughly 0.75 to 3 mm.5
Ultrasound. Ultrasonographic characteristics include a highly reflective, elevated choroidal lesion with a high intensity A-spike and marked shadowing. The B-scan may measure the lesion to be larger than it seemed on funduscopic exam.1
FAF. FAF can be used to document the extent of damage to the RPE. Deossified areas will initially be hyperautofluorescent, as the RPE is stressed and accumulates lipofuscin in the pigment epithelial cells. These areas will then become hypoautofluorescent as RPE atrophy takes place. Hypoautofluorescence in the fovea is associated with subnormal VA.4
FA. FA shows early patchy hyperfluorescence and late diffuse staining and is useful for detecting damage to the RPE and formation of a CNVM.
ICGA. Indocyanine green angiography (ICGA) shows early hypofluorescence and late diffuse multifocal fluorescence.1
OCT. On OCT, choroidal osteomas appear as a sponge- or lattice-like multilayered bony lamellar structure that is transparent to infrared light and thus has minimal optic shadowing. The tumor surface has been described as flat, dome-shaped, or undulating and can be hypo- or hyperreflective. Shields et al. have described findings such as horizontal lamellar lines and other findings on enhanced-depth imaging OCT that may correspond to bony structures such as Haversian canals, Volkmann canals, cement lines, and vascular channels.4 A hyperreflective layer over the RPE on OCT should raise suspicion for development of a CNVM.1 Subretinal fluid may be indicative of RPE dysfunction or CNVM. OCT angiography may allow better visualization of CNVM than FA or ICGA due to blocking from the tumor. OCT angiography can also be helpful in demonstrating regression of CNVM.4
Radiologic imaging such as computed tomography demonstrates a white bone-like lesion in the outer border of the globe. T1-weighted gadolinium-enhanced magnetic resonance imaging demonstrates a high-intensity signal, while T2 weighting shows low intensity.1
Treatment Options
The treatment goal for choroidal osteomas is early detection and treatment of associated CNVM to preserve the patient’s vision. Asymptomatic and peripheral cases should be monitored. Spontaneous resolution of subretinal fluid is possible.
Intravitreal anti-VEGF therapy as a standalone therapy can be used for subfoveal lesions with CNVM and exudation, while a combination of intravitreal anti-VEGF and photodynamic therapy should be considered for extrafoveal lesions. In areas of well-demarcated pigment epithelial leaks on FA, some authors have described success using light treatment with focal argon laser. However, laser therapy must be used with caution, as it may induce the harmful deossification process as well as the atrophy of outer retinal layers, including photoreceptors.6 There are no proven surgical treatments.
Ultimately, the prognosis for visual acuity depends on the lesion’s proximity to the fovea and degree of deossification. Deossified osteomas, particularly those that are subfoveal, have worse visual outcomes.4 The overall likelihood of 20/200 vision or worse has been reported to be as high as 58%.3
Our Patient
We met with Ms. Pearl to discuss the etiology of her CNVM formation. We explained the new diagnosis of choroidal osteoma, along with her poor prognosis. The decision was made to extend the time between appointments and change the treatment schedule to treat as needed.
___________________________
*Patient name is fictitious.
___________________________
1 Kivelä TT. Choroidal Osteoma. In: Rojanaporn D (ed). Ocular Oncology. Springer; 2019.
2 Shields CL et al. Arch Ophthalmol. 2005;123(12):1658-1666.
3 Alameddine RM et al. Middle East Afr J Ophthalmol. 2014;21(3):244-250.
4 Olguin-Manríquez F et al. Int J Retina Vitreous. 2018;4:30.
5 Shields CL et al. Retina. 2015;35(4):750-757.
6 Khan MA. Retina. 2014;34(9):1750-1756.
___________________________
The authors thank Joel Epling, BS, for his significant contribution to this article. Dr. David is a second-year vitreoretinal surgery fellow, Mr. Epling is a third-year medical student, and Dr. Vincent is assistant professor of ophthalmology; all are at Louisiana State University Health Sciences Center in New Orleans. Financial disclosures: None.