By Manuj Kapur, MD, Juan Posada, MD, Shelley Day Ghafoori, MD, Dan S. Gombos, MD, FACS, and Patricia Chévez-Barrios, MD
Edited By: Ingrid U. Scott, MD, MPH
Download PDF
Robin West* is a 75-year-old man with a smoking history of 40 pack years. Because he had an episode of bilateral presumed ocular histoplasmosis syndrome (POHS) 20 years ago, he began to worry about recurrence when he noticed blurred vision in his right eye at both near and distance. With worsening vision over the last three months in his right eye, he visited an eye specialist, who diagnosed him with POHS. He was referred to our eye clinic for further evaluation.
We Get a Look
We confirmed Mr. West’s history of POHS as well as findings of a right lung mass that had increased in size over the previous year.
Vision exam. On examination, Mr. West’s best-corrected visual acuity was 20/70 in the right eye and 20/20 in the left. His intraocular pressure was 12 mm Hg in the right eye and 14 mm Hg in the left. There was no afferent pupillary defect, and his ocular motility was intact.
At the slit lamp. The slit-lamp exam showed no flare or cells in either anterior chamber. Nuclear sclerosis (3+) was apparent in the lens of each eye. There were trace anterior vitreous cells in the right eye and none in the left eye.
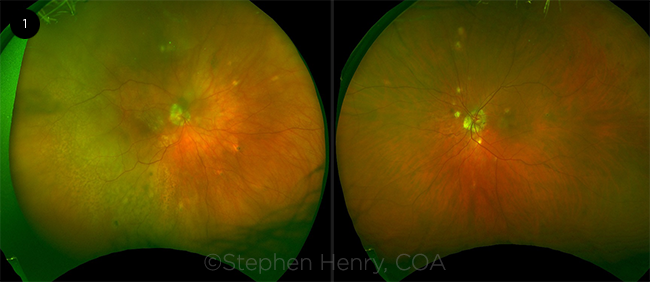
Dilated fundus exam. The dilated fundus exam of the right eye showed yellowish creamy multifocal choroidal lesions in the posterior pole with retinal pigment epithelium (RPE) mottling and choroidal thickening involving the macula (Fig. 1). There was also diffuse temporal choroidal thickening with associated exudative retinal detachment (RD) in the right eye. The fundus exam of both eyes also showed small punched-out chorioretinal scars in the midperiphery.
Imaging. Ocular coherence tomography (OCT) of the macula showed choroidal thickening and subretinal fluid in the right eye and normal exam in the left eye. B-scan showed temporal thickened, hypoechoic choroid in the right eye. Fluorescein angiogram showed mottled punctate hypofluorescence over the choroidal lesions in the right eye, and late staining of midperipheral scars in both eyes.
 |
|
THE RIGHT EYE. During the fundus exam, we noticed yellowish choroidal lesions in the posterior pole of the right eye, with RPE mottling and choroidal thickening.
|
Differential Diagnosis
Clinically, our patient presented with creamy-yellow multifocal choroidal lesions, exudative retinal detachment, and minimal vitritis in his right eye. These signs were very concerning for intraocular lymphoma. However, given Mr. West’s prior history of a right lung mass that had increased in size based on prior chest computed tomography (CT), we were also concerned about metastatic lung cancer. At this point, white dot syndromes and malignant melanoma were low on our differential.
 |
|
IMAGING. OCT showed choroidal thickening and exudative RD.
|
What the Tests Revealed
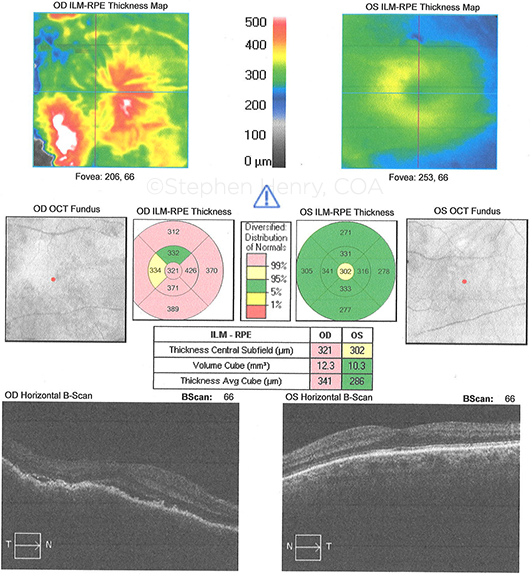
MRI. Initially, magnetic resonance imaging (MRI) of the brain and orbits, with and without contrast, confirmed a right choroidal mass with extraocular extension in the posterior temporal aspect of the globe (Fig. 2). No intracranial spread was noted.
PET-CT. Next, Mr. West underwent a positron emission tomography–computed tomography (PET-CT) scan, which showed no evidence of uptake elsewhere.
Biopsy. Mr. West also had CT-guided biopsy of the right lung nodule due to concern for metastasis. This showed hyalinized fibrous tissue and was negative for lung cancer.
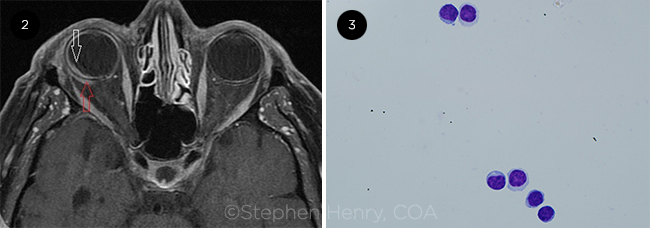
With only ocular involvement, we proceeded with a diagnostic vitrectomy and choroidal biopsy; this showed kappa-positive CD5-positive low-grade monoclonal B cells in the choroid. Immunocytochemistry and flow cytometry showed CD5-positive monoclonal B cells. The vitreous biopsy also showed rare small lymphocytes, predominantly T cells, reactive with rare B cells (Fig. 3). These results were pointing toward a diagnosis of intraocular lymphoma.
Next, we needed to determine whether it was a primary or secondary lymphoma. So, we referred our patient to a hematologist/oncologist in order to rule out concurrent systemic lymphoma. A complete blood count (CBC) was done; this showed normal morphology and no evidence of increased lymphocytes. Mr. West also had a bone marrow biopsy, which did not show evidence of systemic disease.
Our diagnosis. With the combination of minimal vitreous cells, creamy-yellow multifocal choroidal infiltrates, small low-grade lymphoid B-cells detected during biopsy of a thickened choroid, extraocular spread, and absence of clinical evidence of systemic disease, we reached a diagnosis of primary choroidal lymphoma, extranodal marginal zone B-cell subtype.
 |
|
FURTHER TESTING. (2) MRI of the orbits shows extraocular spread. (3) Vitreous cytology shows small lymphocytes (Giemsa stain; 100X original magnification).
|
About the Disease
Intraocular lymphomas are broadly categorized as primary or secondary. Secondary intraocular lymphoma is due to disseminated systemic lymphoma, usually diffuse large B-cell subtype, and commonly involves the choroid.
PIOL. Primary intraocular lymphoma (PIOL) only involves the eye and is mostly non-Hodgkin B-cell subtype. The mean age at presentation is the fifth or sixth decade of life.1
Etiology. PIOL’s etiology is unknown, but several hypotheses have been suggested. Burkitt lymphoma, Epstein-Barr virus (EBV), human immunodeficiency virus (HIV), human T-cell lymphotropic virus (HTLV), and Helicobacter pylori, along with genetic mutations, have been implicated in the lymphoma genesis of various histologies.2
Two subtypes. PIOL can be subdivided into two subtypes: primary uveal lymphoma and primary vitreoretinal lymphoma (PVRL).
Primary uveal lymphoma. Primary uveal lymphoma is usually low grade with a good prognosis and is subdivided into three types: choroidal, iridal, and ciliary body lymphoma.
Primary choroidal lymphoma, which we diagnosed in Mr. West, accounts for the majority of uveal lymphoma cases. Primary choroidal lymphoma is typically unilateral, with diffuse choroidal thickening and yellowish creamy choroidal infiltrates without vitritis. With progression, there is diffuse thickening of the uveal tract as seen on B-scan as well as associated exudative RD. Fluorescein angiogram shows early hypofluorescence corresponding to choroidal infiltrates and late staining of RPE changes. Extraocular involvement, including episcleral extension, is common. The episcleral extension appears as a nonmobile orange to yellowish color lesion or “salmon” patch. This overlapping involvement with ocular adnexal structures is an important component of primary choroidal lymphoma and alerts the clinician to look for coexisting uveal disease if one detects adnexal lymphoma during the exam.3 Biopsy of these sites and choroid can also help in making the diagnosis.
Primary vitreoretinal lymphoma. PVRL is typically bilateral, but frequently asymmetric. It is a high-grade variant of primary central nervous system lymphoma (PCNSL) and has a poor prognosis. Patients with PVRL typically present with vitritis and creamy lesions with orange-yellow infiltrates between RPE and Bruch’s membrane. This gives rise to the characteristic “leopard skin” pigmentation overlying the mass.
Approximately 42%-92% of individuals with PVRL ultimately develop central nervous system (CNS) involvement with a mean interval of eight to 29 months.1 In contrast, 25% of patients with PCNSL will have concomitant PVRL.4 MRI is more sensitive than CT for detecting lymphomatous lesions in the CNS. PET-CT can also help in identifying CNS lesions and ocular activity.
Due to high correlation between PVRL and PCNSL, all patients diagnosed with the ophthalmic disease should undergo a systemic evaluation by an oncologist. This should include MRI of the brain and orbits, cerebrospinal fluid (CSF) studies, CBC, and PET-CT of the chest, abdomen, and pelvis. If PCNSL is also present along with classic ophthalmic findings, a biopsy of the ophthalmic site may not be required. However, in the absence of CNS disease, tissue biopsy of the vitreous and sub-RPE space is the gold standard. Once tissue is obtained, histologic and immunocytochemistry/flow cytometry are performed.
Diagnosing PIOL. Histologically, most lymphomas arise from B cells that exhibit minimal basophilic cytoplasm and elevated nucleus:cytoplasm ratio with hypersegmented nuclei and prominent or multiple nucleoli. Immunocytochemistry and flow cytometry also aid in differentiating various lymphomas. A systemic workup is essential before reaching a diagnosis of PIOL. It is useful not only for staging but also for ruling out secondary or systemic lymphoma with intraocular spread.
Treating PIOL. Currently, ocular radiation or intravitreal methotrexate or rituximab is used to treat isolated unilateral PIOL. When the CNS is involved, high-dose systemic methotrexate as a single agent or as part of a combination regimen is used most commonly.3 The primary choroidal low-grade extranodal marginal B-cell lymphomas have an indolent course and may be treated with local radiation or chemotherapy.
The reported mortality rates from PIOL in the literature are variable. However, one series found a survival advantage if PIOL was diagnosed before CNS involvement—60 months survival versus 35 months.5
Our Patient’s Progress
After detailed discussion, Mr. West elected to undergo local radiation (30 Gy) to the right orbit. At 21 months, the extraocular extension is undetectable on imaging. He continues to come in for periodic systemic surveillance.
___________________________
*Patient name is fictitious.
___________________________
1 Sagoo M et al. Surv Ophthalmol. 2014;59(5):503-516.
2 Devita VT et al. Cancer: Principles and Practices of Oncology. 9th ed. Lippincott, Williams & Wilkins; 2011:1806-1818.
3 Aronow M et al. Ophthalmology. 2014;121(1):334:341.
4 Hochberg FH et al. J Neurosurg. 1988;68(6):835-853.
5 Hormigo A et al. Br J Haematol. 2004;26(1):202-208.
___________________________
Dr. Kapur is staff ophthalmologist and Dr. Posada is chief of hematology-oncology at the Central Texas Veterans Health Care System in Temple, Texas. Dr. Day Ghafoori is clinical assistant professor at the University of Texas (UT) at Austin Dell Medical School. Dr. Gombos is professor and section chief of ophthalmology at the UT MD Anderson Cancer Center in Houston. Dr. Chévez-Barrios is professor of pathology and genomic medicine at the Houston Methodist Hospital.