By Abid Haseeb, MD, Kimberlee Curnyn, MD, and Peter W. Macintosh, MD
Edited by Ahmad A. Aref, MD, MBA
Download PDF
Mona Morley,* a 17-year-old girl, experienced vision loss in her left eye. She initially kept this to herself, but after two months, with the problem getting worse, she confided in her parents. They took her to the community ophthalmologist, who noted that Mona’s vision was hand motion with a relative afferent pupillary defect (RAPD). The ophthalmologist ordered magnetic resonance imaging (MRI) of the brain and orbits, which were interpreted as normal. Mona was then referred to our clinic for further evaluation.
We Take Her History
When we first saw Mona, her medical history included anxiety and depression. She also had been treated for Lyme disease four years previously. Her ocular history included dry eye syndrome, meibomian gland dysfunction, and punctate keratitis in both eyes. Her medications included lamotrigine and venlafaxine for anxiety and depression, and levonorgestrel/ethinyl estradiol for contraception. There was no family history of significant eye disease, autoimmune disease, or episodes of sudden vision loss. Mona stated that she was sexually active with males and always used barrier protection.
She said that the vision loss started two months earlier as a “black splotch” in the middle of her vision in her left eye. The splotch began centrally and spread peripherally. She told us that her left eye had always been the weaker eye. She said that she hadn’t been experiencing headaches, pain with eye movements, or photosensitivity during this two-month period. From the onset of her symptoms, she said that it took a few weeks for her vision to decline to hand motion. During those last few weeks before we saw her, it remained consistently poor.
The Exam
On general examination, Mona was alert and oriented; her mood and affect were appropriate under the circumstances.
In her right eye, visual acuity was 20/20, and color vision, motility testing, confrontation field testing, and pupillary exam were within normal limits.
In her left eye, central vision was reduced to hand motion; she saw 0/11 Ishihara plates; and she had constrictedconfrontation visual fields with an RAPD. Motility was normal.
Intraocular pressures and the slit-lamp exam were normal in both eyes.
In the right eye, the dilated fundus exam revealed a cup-to-disc ratio of 0.3 with a sharp disc margin, absence of pallor and edema, a flat macula, and normal vasculature. In the left eye, cup-to-disc ratio was 0.5 with a sharp disc margin, no edema, and global pallor. The macula was flat, and the retinal vasculature and periphery were normal.
Humphrey 24-2 visual field testing revealed full fields in the right eye and unreliable results in the left. Follow-up Goldmann visual field testing in the left eye showed just a few scattered responses.
A Second Look
The clinical findings of RAPD and painless visual loss suggested optic neuropathy. However, MRI of the brain and orbit performed one week earlier was interpreted by a radiologist as normal, with no evidence of acute ischemia, intracranial hemorrhage, space-occupying lesions, edema, or lesions of the optic nerves. However, on our review of the MRI, we noticed some subtle enhancement of the left optic nerve. We ordered a repeat MRI of the brain and orbits (Fig. 1). MRI of the brain was within normal limits. But on MRI of the orbits, the left optic nerve had increased in size with enhancement, consistent with a diagnosis of left optic neuritis. The rest of the MRI orbit findings were within normal limits.
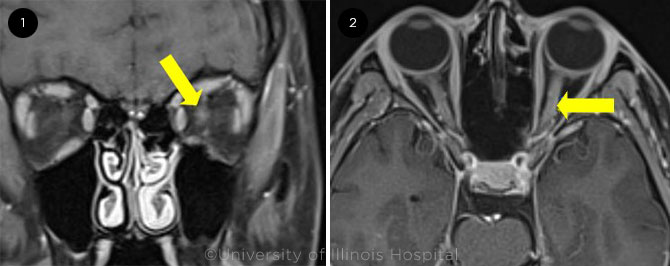 |
|
A SECOND MRI. T1-weighted, fat-suppressed, contrast-enhanced axial MRI of the orbits in (1) coronal and (2) axial views. Left optic nerve enhancement noted (yellow arrows).
|
Differential and Workup
Differential diagnosis. Given clinical findings of unilateral painless vision loss and imaging findings consistent with optic neuritis, we developed a broad differential. Possible autoimmune etiologies included multiple sclerosis (MS), neuromyelitis optica (NMO), Sjögren syndrome, and sarcoidosis. Possible infectious etiologies included HIV, syphilis, cat-scratch disease, John Cunningham virus, adenovirus, Epstein-Barr virus, and varicella zoster virus.
What we ordered. We recommended an inpatient admission for thorough workup of autoimmune and infectious etiologies. A lumbar puncture was performed, and cerebrospinal fluid (CSF) studies were obtained. Because of Mona’s poor spontaneous visual recovery, an MRI of the spine was ordered to investigate for a demyelinating process indicating multiple sclerosis. Inflammation control and immunosuppression can promote vision recovery in the acute phase of optic neuritis. Even though she was no longer in the acute phase, she received 1 g of IV methylprednisolone treatment daily for three days, but this didn’t improve her vision.
Workup’s findings. Mona’s workup revealed the presence of antiaquaporin-4 (AQP4) antibody, also known as NMO antibody. There was also a positive Lyme antibody, consistent with the fact that she had previously undergone treatment for Lyme disease, although she had no symptoms consistent with the disease. MRI of the spine was unrevealing for evidence of demyelination. The rest of the workup, including CSF studies, was within normal limits.
Based on the clinical picture, imaging studies, and serological results, we diagnosed Mona with NMO.
Treatment and Follow-Up
Mona was discharged from the hospital on an oral steroid taper and started on treatment with rituximab. She was also started on prophylactic trimethoprim-sulfamethoxazole. At a three-month follow-up phone conversation, she said that her vision had been stable, and she reported no new symptoms.
Discussion
NMO is an autoimmune inflammatory disease that leads to demyelinating lesions and, when untreated, consequential vision loss and paralysis. A major development in NMO research was the finding of a detectable serum immunoglobulin G (IgG) against AQP4, a channel that regulates fluid homeostasis across the blood-brain barrier.1
Epidemiology. NMO predominantly presents in women in the fourth or fifth decade of life, though one large study found that 5% of AQP4-IgG–positive patients are younger than 18.2 That same study found AQP4-IgG to be seven times more prevalent in females than in males. In terms of demographics, one study reported that 37% of pediatric NMO patients were White, 37% were Black and 13% were Hispanic/Latinx; the frequency of non-White race in that study (63%) was greater than in MS (39%), which predominantly affects White patients.3
Symptoms. Clinically, NMO presents with optic neuritis and transverse myelitis with poor recovery. Presenting symptoms in pediatric NMO include vision loss, motor deficiencies, and constitutional symptoms such as fevers, hiccups, and seizures. A large case series of NMO spectrum disorders in pediatric patients found that 65% presented with optic neuritis, 55% with spinal cord involvement, and 13% involved both.3
NMO in pediatric patients. Most of the clinical, imaging, and laboratory findings in pediatric-onset NMO are similar to those in adult-onset disease. However, in pediatric patients, the female preponderance is lower; there is longer time to increased disease severity; there is a longer time to first treatment; a monophasic disease course is more common; and MRI lesions associated with acute myelitis may be less specific for NMO spectrum diseases.1,4
Making the diagnosis. When NMO is suspected, an appropriately detailed history and physical exam should be obtained. This should be followed by a workup that includes hematologic and metabolic studies, cerebrospinal fluid studies, serologic studies for antibodies associated with autoimmune etiologies, and evidence of infectious etiologies underlying vision loss. For patients with AQP4-IgG seropositivity, a diagnosis of NMO can be confirmed—according to consensus diagnostic criteria from the International Panel for NMO Diagnosis—if at least one core clinical characteristic (such as optic neuritis or myelitis) is present and alternative diagnoses can be excluded.1 Our patient satisfied these criteria. In patients without confirmed AQP4-IgG, there are additional diagnostic requirements: The patient should have at least two core clinical characteristics and, to enhance diagnostic specificity, MRI scans should show supportive characteristics. For example, if one of the core clinical characteristics is acute myelitis, a longitudinal MRI scan that shows a lesion extending over three or more contiguous segments would be needed.1
Treatment. Treatment of NMO focuses on minimizing disease progression by mitigating acute attacks and preventing future exacerbations. Treatment of acute attacks involves the use of IV methylprednisolone. Since 2019, three new targeted therapeutic agents for NMO—eculizumab (complement C5), inebilizumab (CD19+ B cells), and satralizumab (interleukin-6)—have become available and are FDA-approved for first-line therapy of patients with this disorder. Newly diagnosed patients should be considered for one of these agents. Additionally, some mainstays of MS treatment, namely interferon-beta and natalizumab, may increase relapse rate in NMO, underscoring the importance of distinguishing between these two disease processes.5
Prognosis. One study of 106 patients with AQP4-IgG–positive NMO found that, after a median disease duration of 75 months, 18% of patients developed permanent bilateral visual disability, 34% had permanent motor disability, and 23% had become wheelchair-dependent.6 In the small number of patients who were treated before their first relapse, none developed permanent visual disability. However, early treatment did not protect against motor disability. Patients with monophasic disease courses were treated earlier than patients with relapsing disease (three months vs. 54 months). While the understanding around prognosis in NMO-spectrum diseases is developing, these findings suggest that delays in treatment portend worse disease courses. Timely diagnosis and treatment are critical.
Conclusion
This case emphasizes the importance of putting NMO on the differential diagnosis for vision loss, even in pediatric patients, who may experience significant vision loss before reporting it to parents. Furthermore, it highlights the need for ophthalmologists to thoroughly re-interrogate the findings and assumptions in referred cases because the finding of optic neuritis may initially be missed, as it was in this instance. Imaging alone may not be sufficient for diagnosis, and clinicians should be aware of the laboratory testing available for a thorough workup.
___________________________
* Patient name is fictitious.
___________________________
1 Wingerchuk DM et al. Neurology. 2015;85(2):177-189.
2 Quek AM et al. Arch Neurol. 2012;69(8):1039-1043.
3 Chitnis T et al. Neurology. 2016;86(3):245-252.
4 Collongues N et al. Neurology. 2010;75(12):1084-1088.
5 Trebst C et al. J Neurol. 2014;261(1):1-16.
6 Kitley J et al. Brain. 2012;135(1);1834-1849.
___________________________
Dr. Haseeb is in his internship year at the University of Illinois Hospital in Chicago. Dr. Curnyn and Dr. MacIntosh are at the Illinois Eye and Ear Infirmary in Chicago; Dr. Curnyn is clinical assistant professor of ophthalmology in the Pediatric Ophthalmology and Adult Strabismus Service, and Dr. MacIntosh is assistant professor of ophthalmology and director of the Residency Program. Financial disclosures: Mr. Haseeb and Dr. Curnyn: None. Dr. MacIntosh: NEI: S; Research to Prevent Blindness: S.
See the disclosure key at www.aao.org/eyenet/disclosures.