Download PDF
The human gut houses up to 70% of the immune system. Within the gastrointestinal tract live up to 100 trillion organisms, an ecosystem linked to both innate and adaptive immune function in animal models.1,2
The Human Microbiome Project, propelled by advances in high-throughput 16S RNA gene sequencing, has amassed evidence of the gut microbiome’s impact on a host of immune-mediated diseases, including multiple sclerosis, type 1 diabetes, rheumatoid arthritis, ankylosing spondylitis, and cardiovascular disease.1 And in ophthalmology, recent research is looking into the link between the gut microbiome and the pathogenesis of ocular disease.
In today’s common usage, “microbiome” refers to the community of bacteria, viruses, fungi, and yeast—plus all their genetic material—that live in or on the body. “Microbiota” refers to individual microbes themselves.1
Just how complex is the gut microbiome? An estimated 1,000 species of bacteria alone live commensally in the human digestive system, with 10 times the number of genes in the human genome.3 Only a few of these microbes are well understood. It’s as if, in a vast orchestra, only a handful of instruments can be heard. But these early notes may lead to new therapeutic paradigms where the gut microbiome plays a role in treating eye disease.
 |
|
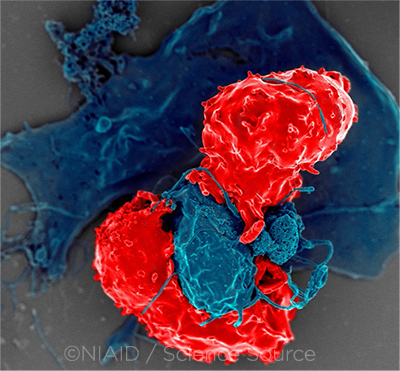
INFLAMMATION. Noninfectious uveitis is known to be characterized by an imbalance between specific types of T cells, including the regulatory T cells (Tregs) that normally prevent autoimmune diseases. This image shows Tregs interacting with antigen-presenting cells.
|
Uveitis: The Most Studied Link
Microbiota and gut permeability. Compelling evidence links the gut microbiome with uveitis in animal models of experimental autoimmune uveitis (EAU) and mice with spontaneous uveitis.4 Noninfectious uveitis is now known to be characterized by an imbalance in the ratio of autoreactive, pathogenic effector T cells—including T helper (Th1) and Th17 cells—to the regulatory T cells (Tregs) that normally prevent autoimmune diseases from occurring.4,5
“Ocular inflammatory diseases like uveitis are thought to arise from systemic immune defects or alterations. And intestinal mucosal immunity, it turns out, is a very significant contributor to systemic immunity,” said Phoebe Lin, MD, PhD, at the Casey Eye Institute in Portland, Oregon. “What we and others have found is that not only changes in the intestinal microbiota but also changes in intestinal permeability can be associated with increased severity and possibly pathogenesis of ocular inflammation,” such as that seen in uveitis.
Researchers in Dr. Lin’s lab found that giving mice a dose of short-chain fatty acids (naturally occurring metabolites of intestinal bacteria fermentation of dietary fiber) decreased the severity of uveitis in EAU via two mechanisms: enhancing the Tregs in the colon and cervical lymph nodes, and slowing migration of effector T cells between the gut and spleen.5 In another study, they showed decreased severity of EAU in mice given oral antibiotics. In these mice, the short-chain fatty acids reduced severity of EAU but promoted intestinal bacterial changes that increased Tregs in the gut.6
Retina-specific T cells in the gut. In her lab at the NEI, Rachel R. Caspi, PhD, has shown that retina-specific T cells could be activated in the gut. Her work suggests that microbiota signal retina-specific, autoreactive T cell receptors in the gut, which can lead to uveitis.4 “We showed that lymphocytes are seeing something in the gut that binds to the specific T cell receptor, something that mimics the antigen they would normally recognize in the retina,” Dr. Caspi said.
A paradox led to her research: T cells must first be activated before they can enter the eye, but retinal antigens are not found outside the eye. “What do these lymphocytes see that activates them, given that the antigen they recognize is sequestered behind the blood-retinal barrier?” Dr. Caspi asked. “That’s when we started looking at the gut as the source of potential antigens that may mimic and activate the T cells, through both T cell receptors and the innate receptors every immune cell has,” she said. Her work also suggests that gut microbiota may act as an “adjuvant” that boosts the innate immune system response, leading to uveitis.7
But puzzles remain. “We have not yet found what components stimulate these lymphocytes in the gut—which bacteria and which molecules from the bacteria are involved,” Dr. Caspi said. Her ongoing work studies germfree mice harboring microbiota from healthy human donors and from uveitis patients. “Association is not causation, and results can show a parallel but independent effect,” she said. “It’s necessary to do ‘take away’ and ‘add back’ experiments to conclusively prove causality.”
Observational studies in humans. Chakravarthy et al. compared the gut microbiota of uveitis patients with healthy controls and found that patients had less diversity in microbiota and fewer anti-inflammatory microbes.7
H. Nida Sen, MD, MHSc, at the NEI, has also found that uveitis patients have a gut microbiome different from controls. “It’s hard to tease out the clinical factors that might have an impact on this finding of difference,” said Dr. Sen, who has retooled her latest study to compare treatment-naive uveitis patients with more homogeneous controls matched by age, gender, race, and geographic area. “We also collected detailed dietary information in the hopes of accounting for those differences,” she said. Her lab is currently sequencing data from this study.
“Are these changes there because the disease is creating an environment where proinflammatory bacteria are finding a nice location to prosper—or are they the cause of the disease?” Dr. Sen asked. “It’s very difficult to tease that out in humans.”
In China, Ye et al. found that the gastrointestinal tract of patients with Vogt-Koyanagi-Harada disease (VKH) had a gut dysbiosis that was later corrected with immunomodulatory therapy.8 The researchers’ work led to a VKH classifier, based on 37 differentially depleted or enriched microbes. The classifier distinguishes patients with good prognoses from those unlikely to respond to immunosuppressive therapy.8
Why is this China study important? “They found enrichment of Prevotella species and depletion of Clostridium species—and with immunomodulatory treatment of uveitis, they showed that this gut microbiota shifted toward normal,” said Dr. Sen. In part of the study, the researchers also colonized mice with microbiome taken from patients with VKH before they induced uveitis, “and the mice showed significant increase in disease severity,” she said.8 The clinical takeaway? “It supported the findings that these changes in the gut microbiome in patients were shifting with treatment, predicting treatment response, and recapitulating worsening disease with the fecal transplant from diseased patients into animals,” she said.
 |
|
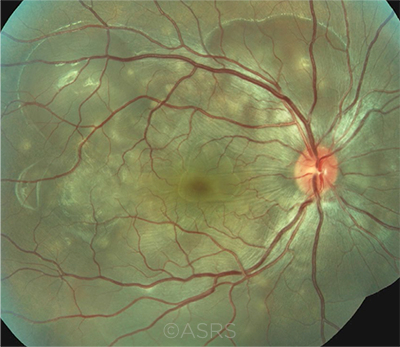
VKH. Researchers have used immunodulatory therapy to correct gut dysbiosis in patients with Vogt-Koyanagi-Harada disease. This work has been used to predict treatment response. This image was originally published in the ASRS Retina Image Bank. Renata Garcia Franco, MD. Vogt-Koyanagi-Harada Disease OD. Retina Image Bank. 2020; Image Number 58107. © The American Society of Retina Specialists.
|
AMD: Evaluating Inflammatory Pathways
The pathogenesis of age-related macular degeneration (AMD) is thought to involve various inflammatory pathways, most notably complement activation. “Alterations in the gut microbiota, or dysbiosis, may activate inflammatory pathways that then result in ocular problems, but it’s not yet cause-and-effect,” said Lisa Park, MD, at Columbia University Medical Center in New York City. A lot of the microbiome research is about “trying to identify the species of bacteria, viruses, parasites, and fungi, and to understand—if we alter the proportions and take them out of equilibrium—whether that’s implicated in the development of certain types of ocular disease,” she said.
In one study involving aging mice, a high-glycemic diet led to degeneration of photoreceptors and atrophy of retinal pigment epithelium cells not seen in mice fed a normal diet.9 Reverting to a low-glycemic diet reversed the features of disease and changed levels of AMD-protective factors, including serotonin, in the gut.9 And in another study, Dr. Lin and her colleagues found changes in the metabolic pathways, represented by intestinal bacteria, in AMD patients compared to controls.10
“We know from the Age-Related Eye Disease Studies that vitamin supplementation slows the progression of drusen,” said Dr. Park. “In some mice you can see alterations in specific bacteria by altering, for example, the zinc in their diet. But whether diet is directly related is a future area of study.”
 |
|
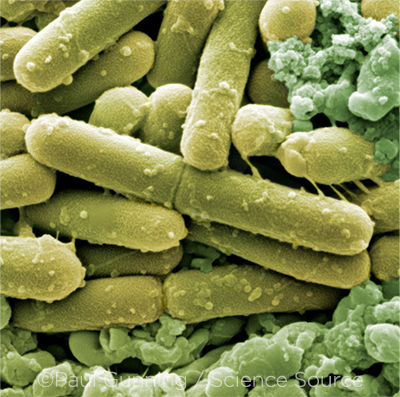
FUTURE TX. Could fecal microbiota transplants eventually be used to treat selected ocular diseases? In gastroenterology, the transplants have been successfully used to eradicate infection with C. difficile bacteria (shown here).
|
Dry Eye: Below the Surface
“There’s a lot of interest in the differences in the gut microbiome in [dry eye] patients and healthy controls,” said Anat Galor, MD, MSPH, at the Miami VA and Bascom Palmer Eye Institute in Miami. “Many differences have been found, but you’re looking at a small number of humans and lots of bugs. You’re bound to find differences, but do they really mean anything?”
A role for fecal transplants? Dr. Galor, who focuses on dry eye, recently completed a fecal microbiota transplant (FMT) study of patients with immune-mediated dry eye. “We gave them two enemas with FMT and followed them for three months,” she said. “Some patients said they felt better; some felt the same. Now we’re looking deeply into the data to ask: Did we change the microbiome with two enemas? Is that enough? Did we see any changes in immune profiles based on that microbiome change?”
Dr. Galor noted that Cintia de Paiva, MD, PhD, and colleagues have found that dry eye is more severe in germfree mice.11 “If you raise germfree mice without bacteria in their gut, they have a more severe dry eye phenotype with corneal staining and surface inflammation,” Dr. Galor said. She added, “What’s really cool about this research is that if the germfree mice are put in a cage with healthy mice, the mice will eat each other’s poop. This recolonizes the gut [of the germfree mice] with bacteria, and their disease gets better.”
 |
|
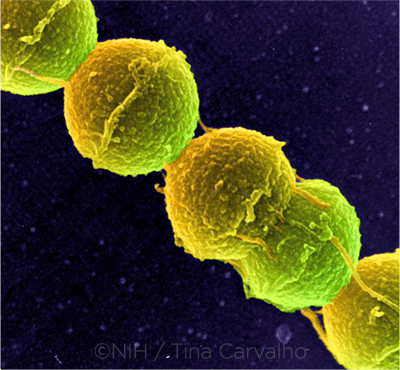
STREP. Streptococcus can occur in pairs or in chains, as shown here. Research on a potential link between streptococci and glaucoma is in the very early stage.
|
Glaucoma: Early Investigations
Other researchers are investigating the association between oral and gut microbiota and the development of glaucoma. For instance, some people with primary open-angle glaucoma have an abundance of bacteria in their oral cavity, particularly streptococci, said Dr. Park. However, she cautioned, “that’s a long way from a direct link to developing glaucoma.”
A possible link: Gut dysbiosis triggers changes in cytokine signaling and complement activation in the immune system.12 In addition, some glaucoma patients have an underlying genetic profile of mitochondrial DNA, perhaps associated with gut microbiome alterations, she said.
Gut-Eye Axis: Working Theories
Research has driven the latest hypotheses on how the gut-eye axis works—and, as the experts noted, these hypotheses aren’t mutually exclusive.
T cell threshold model. “The bacteria that dominate—some are inflammatory, some are anti-inflammatory—activate or down-regulate the immune system,” said Dr. Lin. “This is so specific that certain strains of bacteria promote inflammatory T cell types, like Th17, which are thought to be important in the pathogenesis of certain types of autoimmune uveitis. Data from my lab, and other labs as well, indicate that this differential T-cell induction, based on changes in the intestinal microbiota, results in either protection from or worsened autoimmune uveitis.”
Molecular mimicry model. This model holds that an auto-immune process can be triggered because the immune system recognizes an organism that’s structurally similar to an ocular antigen and then launches a reaction of the immune system against the eye tissue. “Bacteria in the gut appear to mimic that unique retinal antigen and trigger these lymphocytes to become activated,” said Dr. Caspi.
Leaky gut model. “Simply increasing the permeability of the gut epithelial barrier may allow microbial constituents, or bacteria itself, to leak into the bloodstream,” Dr. Lin said. “It’s not a direct infection of the extraintestinal tissue site; the inciting factor is the increase in gut permeability.”
Complement system defects. The complement system plays a key role in the innate immune system. “If there’s an inappropriate amount of intestinal bacteria, then you could have inappropriate complement activation that might lead to diseases like macular degeneration,” said Dr. Lin.
Genetic defects. Some types of uveitis have the same genetic marker, HLA-B27, that has been linked to ankylosing spondylitis. “There are some studies where transgenic HLA-B27 rats are shown to have more gut inflammation, but whether this directly influences disease is another question,” said Dr. Park.
|
Future Therapeutic Approaches
How might gut microbiota be targeted to help treat eye disease?
Antibiotics. The most obvious way to alter the intestinal bacteria is with antibiotics, said Dr. Lin. “Based on experimental data in animals, if you alter the intestinal bacteria with targeted antibiotics, you can dramatically reduce the severity of experimental autoimmune uveitis through bacterial changes in the gut that promote regulatory T cell differentiation.”
High-fiber diet and short-chain fatty acids. “We’re knee-deep in experiments that look at different types of diet that can influence the microbiota beneficially, so that you can reduce ocular inflammation in EAU,” said Dr. Lin. “A high-fiber diet will promote certain bacteria to be more predominant in the gut, and these bacteria produce short-chain fatty acids that promote regulatory T cell differentiation and reduce the propensity to develop ocular inflammation.” A few labs are directly administering short-chain fatty acids to test this as a therapeutic intervention in extraintestinal autoimmune diseases, she said. “In our mice, it was protective for autoimmune uveitis,” Dr. Lin said.
“It turns out it’s very difficult to change your microbiome,” said Dr. Galor. “Someone with a vegan diet for 30 years would have a different microbiome from someone who has eaten meat for a lifetime, but short-term diet changes may not have a meaningful effect.”
“I still recommend patients follow a very healthy diet,” said Dr. Sen. “The high-fiber Mediterranean diet has been repeatedly associated with good health outcomes in general, and we know that a healthy diet can influence many diseases, including AMD.”
Probiotics. Certain strains of bacteria normally found in the gut damp down inflammation. If a healthy diet promotes a balanced, well-colonized microbiome, do patients need probiotics?
“The challenge with prebiotics and probiotics is which one for what disease?” said Dr. Galor. “We have some diseases with specific bacterial signatures and others with a wide range of microbiome alterations.” And with thousands of available products, she said, “there is no clarity on which would be best for a particular disease. Also, as with diet, short-term use of probiotics often doesn’t produce meaningful changes in the microbiome.”
“The ultimate difficulty is that it’s hard to develop a formulation of probiotics that can colonize the gut well enough to have a persistent therapeutic effect,” said Dr. Lin. “Maybe in the future there will be a formulation that colonizes better, or perhaps treatment will entail resetting the microbiota with antibiotics and then recolonizing with the correct type of bacteria using probiotics and diet.” The latter approach “might reset the intestinal, and thus the systemic, immune system,” she said.
Fecal transplants. During an FMT procedure, a healthy person’s stool is harvested and transplanted into the gastrointestinal tract of a person with disease. This is often performed via colonoscopy, although it can be done via a nasogastric tube or an enema.
“We have good data with C. difficile infection, where one FMT is generally successful in eradicating infection, as well as some positive effects with ulcerative colitis,” Dr. Galor said. “We are far from understanding how FMT might be used to treat ocular disease, but perhaps, one day, we could go right to the source, find a healthy donor, and use their microbiome to treat eye disease.”
Interventional trials. Looking ahead, “The next leap will be to an interventional trial altering the gut microbiome,” said Dr. Sen, “either alone or as an adjunct therapy for some forms of eye disease, especially with uveitis and AMD.”
___________________________
1 Lin P. Clin Exp Ophthalmol. 2019;47:418-422.
2 Kodati S, Sen HN. Best Prac Res Clin Rheumatol. 2019;33(6):101500.
3 Nakamura YK et al. Sci Rep. 2017;7(1):11745.
4 Horai R et al. Immunity. 2015;43(2):343-353.
5 Mochizuki M et al. Prog Retin Eye Res. 2013;33:10-27.
6 Nakamura YK et al. Invest Ophthalmol Vis Sci. 2016;57(8):3745-3758.
7 Horai R, Caspi RR. Front Immunol. 2019;10:232.
8 Ye Z et al. Gut Microbes. 2020;11(3):539-555.
9 Rowan S et al. Proc Natl Acad Sci U S A. 2017;114(22):E4472-E4481.
10 Kiang L et al. Invest Ophthalmol Vis Sci. 2017;58(8):5739.
11 Wang C et al. Int J Mol Sci. 2018;19(2):565.
12 Lu LJ, Liu J. Yale J Biol Med. 2016;89(3):325-330.
Meet the Experts
Rachel R. Caspi, PhD Senior investigator in the NEI’s Immunoregulation Section. Relevant financial disclosures: Cua DJ et al., inventors; Merck Sharp & Dohme, assignee. Use of IL-23 and IL-17 antagonists to treat autoimmune ocular inflammatory disease. US patent 8,524,230 B2. Sept. 3, 2013. (No royalties to date.)
Anat Galor, MD, MSPH Staff physician at the Miami Veterans Administration Medical Center and associate professor of ophthalmology at the Bascom Palmer Eye Institute in Miami. Relevant financial disclosures: Allergan: C; Dompé: C; Novaliq: C; Novartis: C; Oculus: C.
Phoebe Lin, MD, PhD Associate professor of ophthalmology at the Oregon Health & Science University’s Casey Eye Institute in Portland. Relevant financial disclosures: Collins Medical Trust: S; NEI: S; NIH: S; Research to Prevent Blindness: S.
Lisa Park, MD Associate professor of ophthalmology at Columbia University Medical Center, New York, N.Y. Relevant financial disclosures: None.
H. Nida Sen, MD, MHSc Lasker Clinical Research Scholar, clinical investigator, and director of the Uveitis Clinic and Uveitis and Ocular Immunology Fellowship Program at the NEI. Relevant financial disclosures: None.
Full Financial Disclosures
Dr. Caspi Cua DJ et al., inventors; Merck Sharp & Dohme, assignee. Use of IL-23 and IL-17 antagonists to treat autoimmune ocular inflammatory disease. US patent 8,524,230 B2. Sept. 3, 2013. (No royalties to date.)
Dr. Galor Allergan: C; Dompé: C; NIH: S; Novaliq: C; Novartis: C; Oculus: C; Research to Prevent Blindness: S; U.S. Department of Defense: S; U.S. Department of Veterans Affairs: S.
Dr. Lin Alcon: S; Collins Medical Trust: S; NEI: S; NIH: S; Oregon Health & Science University: S; Research to Prevent Blindness: S; Thome Foundation: S.
Dr. Park None.
Dr. Sen None.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|