By Roma B. Pegany, MD, and Kenneth L. Cohen, MD
Edited by Ahmad A. Aref, MD, MBA
Download PDF
Divya Diamond,* a 30-year-old White woman, had been noticing a gradual decline in her vision in the right eye over the last month.
Ocular History
At 10 years old, a glaucoma suspect. Ms. Diamond was first seen in our ophthalmology clinic when she was 10 years old. She had been referred by her primary care physician for decreased vision in her right eye. She had a history of attention deficit hyperactivity disorder and well-controlled asthma, and she was recently diagnosed with Sturge-Weber Syndrome (SWS) without leptomeningeal involvement.
At that time, a port-wine stain was noted. She mostly had right-sided facial swelling and underwent treatment with embolization of arteriovenous malformations (AVMs).
The 10-year-old’s best-corrected visual acuity (BCVA) was 20/20 in each eye, and cycloplegic refraction showed mild myopia in the right eye only. Her glaucoma specialist diagnosed her as a glaucoma suspect based on family history, slight cup-to-disc asymmetry (0.4 in the right eye and 0.3 in the left), and high-normal IOPs. Her retinal exam was otherwise normal without evidence of a choroidal hemangioma.
Further testing was planned, but she was lost to follow up.
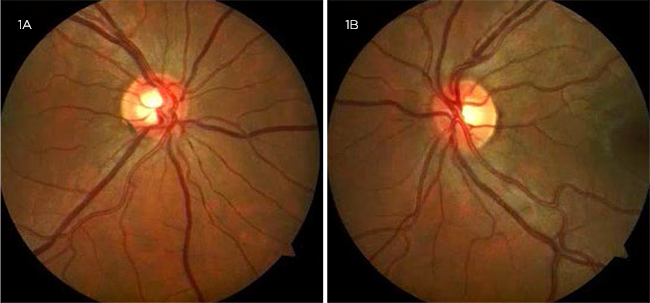
At 27 years old, open-angle glaucoma. Ms. Diamond returned to our clinic 17 years later for routine monitoring. Records showed that she had been seen by an ophthalmologist only once in the interim at an outside office. Her vision at follow-up with us remained unchanged. Her IOP was 17 mm Hg and 13 mm Hg in the right and left eyes, respectively. Gonioscopy showed that her angles were open to ciliary body band bilaterally with no blood in Schlemm’s canal. Central corneal thickness was 540 and 565 μm in the right and left eyes, respectively. Her cup-to-disc ratio showed increased asymmetry with 0.7 in the right eye and 0.45 in the left eye (Fig. 1). Although OCT of the peripapillary retinal nerve fiber layer was within normal range, progressive thinning was found when compared to imaging from the outside office. On this basis, and in the setting of reportedly increased IOPs at the outside office, a diagnosis of secondary open-angle glaucoma due to elevated episcleral venous pressure was made. She was started on latanoprost but was lost to follow-up.
 |
|
MS. DIAMOND AT 27. Her cup-to-disc ratio showed increased asymmetry.
|
We Get a Look
At 30 years old, a sudden change. At age 30, Ms. Diamond presented to our ophthalmology urgent care clinic for a one-month history of decreased vision in her right eye. She said that her distance vision seemed blurred, but there had not been any shadows or flashes. She also shared that her right pupil seemed “dilated” and that she had not been taking her eyedrop. She said that there had not been any trauma. She had been experiencing worsening right facial AVMs and was undergoing sclerotherapy treatment using bleomycin injections of her upper lip.
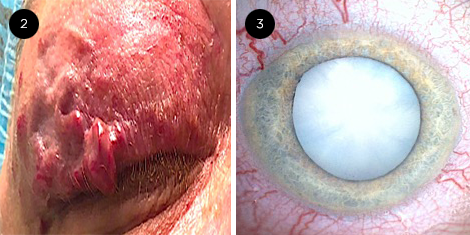
Ophthalmic examination. On examination, Ms. Diamond’s VA was counting fingers in the right eye and 20/20 in the left eye. Her IOP was 9 mm Hg and 13 mm Hg in the right and left eyes, respectively. She had a fixed 7-mm pupil in the right eye and a normal pupil in the left eye. There was no reverse afferent pupillary defect (APD), and motility was normal. Her external exam was significant for right facial AVMs that involved the right upper eyelid (Fig. 2). A slit-lamp examination of the right eye showed prominent episcleral and scleral blood vessels and an intumescent mature cataract (Fig. 3). The fundus of the affected eye could not be seen, and a B-scan ultrasound was within normal limits. Examination of the unaffected left eye was normal. To potentially improve Ms. Diamond’s vision, cataract surgery was recommended.
The cataract surgery. Because of the intumescent, pressurized lens, we planned to use the Brazilian technique.1 This is a two-step technique. Step one equalizes the pressure between the anterior chamber and the anterior lenticular compartment using a needle incision of the anterior capsule and injection of a low viscosity ophthalmic viscoelastic device. After a small capsulorrhexis is created, step two equalizes the pressure between the anterior and posterior intralenticular compartments via use of bimanual irrigation and aspiration (I&A) to move the nucleus. This creates communication of the compartments at the equator of the nucleus.
However, when we created the temporal corneal incision, the right shoulder of the keratome nicked the anterior capsule, creating the Argentinian flag sign (see the video). We used a cystitome and Giannetti capsulorrhexis forceps to complete the capsulorrhexis. Hydrodissection was not performed. Via the use of coaxial I&A, the cataract was carefully wiggled and rotated to break any equatorial block, and the cataract was aspirated. The tear did not extend posteriorly, and a one-piece IOL was placed in the capsular bag with the haptics away from the tear. Her postoperative course was uncomplicated, and her BCVA improved to 20/30.
Follow up. We saw Ms. Diamond a few weeks later for glaucoma monitoring. She was happy with her improved vision, but she pointed out that her pupil remained dilated. The combination of a white cataract with a fixed pupil without an APD could be suspicious for trauma. The rest of Ms. Diamond’s exam, including IOP, was stable. Currently she is off drops and being monitored. She follows closely with her interventional radiologist and is now receiving sclerotherapy with bleomycin for her eyelid AVMs.
 |
|
MS. DIAMOND AT 30. When she was seen at 30 years old, (2) we noted right facial AVMs involving the upper eyelid and (3) an intumescent mature cataract in the right eye.
|
Discussion
SWS is a rare, congenital neuro-oculocutaneous disorder that was first reported in 1879 in a 6-year-old girl.2 Unlike most other phakomatoses, such as neurofibromatosis and tuberous sclerosis, SWS is not inherited, although a somatic mutation in the GNAQ gene has been identified.2 A mutation in this gene leads to the development of abnormal blood vessels. Timing of this mutation during development impacts clinical phenotype, with a facial port-wine stain in the ophthalmic division of the trigeminal nerve being the classic vascular malformation associated with SWS. However, SWS may be associated with varied facial angiomas, as well as angiomas involving the leptomeninges or the upper eyelid.
Glaucoma. The most common ocular manifestations associated with SWS are glaucoma and choroidal hemangiomas. The incidence of glaucoma ranges from 30%-70%.2 The age of presentation of glaucoma shows three peaks: during the first year (40%), between 5 and 9 years of age (23%), and after the age of 20 (20%).2 Hence, patients with SWS require continued ophthalmologic monitoring even if they remain asymptomatic during childhood. Developmental angle anomalies and elevated episcleral venous pressure are thought to be the cause of glaucoma.3 Initial treatment is with medical management; however, because SWS glaucoma can be refractory, it may require surgical intervention.
Choroidal hemangiomas. Diffuse choroidal hemangiomas are another common ocular manifestation of SWS. Typical findings show a generalized red-orange choroidal thickening, which gives the appearance of a “tomato ketchup” fundus. Vision loss may occur from refractive error, foveal distortion, or exudative retinal detachment.4 Successful treatment has been shown with photodynamic therapy and low-dose external beam radiotherapy for exudative retinal detachments.4
An Unusual Finding
Unlike glaucoma and choroidal hemangiomas, cataracts are an unknown ocular manifestation of SWS. They are usually described as a complication of glaucoma surgery and not as a primary finding in this syndrome. To the best of our knowledge, this is the first case in the English-language literature of a mature, white, intumescent cataract in a young adult patient with SWS. The intumescent cataract requires a specific operative technique to avoid the complication we experienced in Ms. Diamond’s case.
Only two other case reports describing cataracts in children with SWS have been published. In one case, a 6-year-old girl presented with hand motion vision due to a mature cataract.5 In a second case, a 5-year-old girl presented with no light perception vision with findings of a dense vitreous hemorrhage, choroidal hemangioma, and cataract.6 In the latter case, the authors thought that perhaps ocular siderosis from extensive and persistent vitreous hemorrhage led to cataract formation.
Although the exact mechanism for cataract development in our patient could not be identified, the predisposition may be a consequence of the underlying SWS and/or bleomycin treatment, especially given the constellation of unilateral findings. Further studies are necessary to elucidate the mechanism by which cataracts, which can cause significant visual loss, may be related to SWS.
Conclusion
As demonstrated in Ms. Diamond’s case, the prediction of clinical outcomes remains challenging in patients with SWS. Therefore, lifelong monitoring remains the cornerstone in management of this rare condition.
___________________________
*Patient name is fictitious
___________________________
1 Figueiredo CG et al. J Cataract Refract Surg. 2012;38(9):1531-1536.
2 Sudarsanam A et al. Eur J Paediatr Neurol. 2014;18(3):257-266.
3 Aggarwal NK et al. J Pediatr Ophthalmol Strabismus. 2010;47(6):361-365.
4 Silverstein M et al. Curr Opin Ophthalmol. 2019;30(5):301-305.
5 Trivedi HL et al. Bombay Hospital Journal. 2008;50(2):310-315.
6 Geraldo AF et al. Neuroradiol J. 2012;25(1):85-88.
___________________________
Dr. Pegany is a third-year ophthalmology resident at the University of North Carolina (UNC), Chapel Hill. Dr. Cohen is the Sterling A. Barrett Distinguished Professor of Ophthalmology at UNC Kittner Eye Center in Chapel Hill. Financial disclosures: None.