By Ryan Jaber, BS, and Carol L. Karp, MD
Edited by Ingrid U. Scott, MD, MPH, and Sharon Fekrat, MD
This article is from March 2012 and may contain outdated material.
Ocular surface squamous neoplasia (OSSN) comprises a wide spectrum of dysplastic changes to the epithelium of the cornea, limbus and conjunctiva. These changes include corneal and conjunctival intraepithelial neoplasia (CIN) and squamous cell carcinoma (SCC).
According to the ocular surface classification model used for OSSN, the dysplasia in CIN is confined to the epithelium and does not invade below the basement membrane. In contrast, SCC invades through the basement membrane into the deeper layers.
Although OSSN is the most common neoplasm of the ocular surface, estimates of its incidence vary significantly. With regard to recurrence, the rate after surgical excision has been reported to be as low as 5 percent with clear margins and, in one study, as high as 56 percent with positive margins.1
Etiology
The dysplastic cells in OSSN are thought to arise from limbal stem cells. Known predisposing factors for OSSN include ultraviolet light exposure, advanced age, fair skin, a history of heavy smoking and a history of exposure to petroleum-based products. In addition, an increased incidence of OSSN has been found in people with xeroderma pigmentosum and HIV infection; the latter is especially common in younger patients with OSSN.
It has been theorized that the human papillomavirus (HPV) could play a role in OSSN; however, that possible role has yet to be clearly defined.
|
|
|
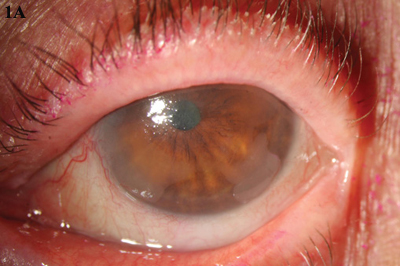
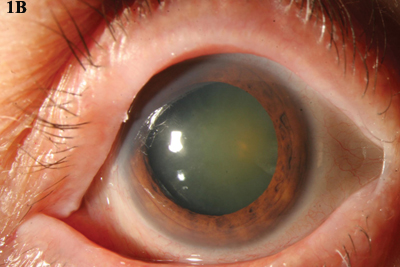
MMC. (1A) An opalescent CIN lesion, visible at 4 and 8 clock hours, was treated with four cycles of topical MMC drops. (1B) Complete resolution was achieved.
|
Signs and Symptoms
The typical OSSN lesion is an elevated, thickened, grayish plaque with a leukoplakic, gelatin-like or papillomatous appearance and accompanying blood vessels. Patients usually present with a unilateral lesion, and they often complain of redness and irritation in that eye. These lesions typically are located in the limbal region within the interpalpebral zone, where the ocular surface is exposed to UV radiation. When an OSSN lesion is located on the cornea, it has an opalescent appearance and may be overlooked as a pannus.
|
|
|
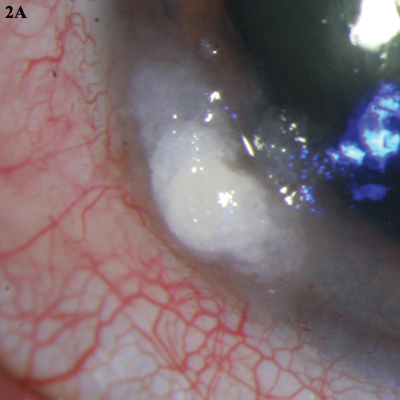
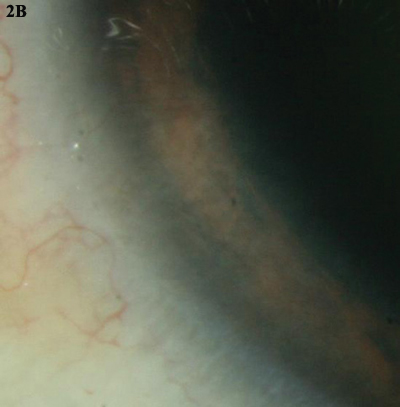
5-FU. (2A) This leukoplakic CIN was treated with three cycles of topical 5-fluorouracil. (2B) The lesion completely resolved.
|
Treatment Evolves
The traditional treatment for OSSN is surgical excision with wide margins of about 4 mm and adjuvant cryotherapy. However, the large excision area can lead to limbal stem cell deficiency, ocular surface irregularity and scarring.
Recently, primary treatment with topical chemotherapy—mitomycin C (MMC), 5-fluorouracil (5-FU) or interferon alpha-2b (IFN-a2b)—has emerged as an alternative. Advantages include less-invasive treatment of extensive lesions and the ability to target “invisible” or subclinical disease.
In addition to using topical chemotherapy as a primary treatment, the surgeon can use it for larger tumors preoperatively to shrink the tumor and facilitate excision. It also may be employed postoperatively to treat positive margins or recurrent disease.
MMC. MMC is isolated from Streptomyces caespitosus and, after activation, is used as a chemotherapeutic and antibiotic agent because of its alkylating properties. MMC forms free radicals that interact with DNA, resulting in strand breakage and impaired DNA synthesis. Because of the rapid turnover and oxidation rate of tumor cells, they are more vulnerable to MMC than normal cells.
Overall, topical MMC works well in eradicating OSSN (Figs. 1A and 1B), with an efficacy rate of 70 to 100 percent.2 Typical primary therapy consists of 0.02 percent to 0.04 percent MMC given as eyedrops four times daily, with cycles of one to two weeks “on” and one to two weeks “off.”
MMC has a higher incidence of reported side effects than 5-FU and interferon, which may lead to the discontinuation of therapy. Pain, redness and severe epitheliopathy are common. Limbal stem cell deficiency is a significant long-term risk; consequently, epithelial defects are frequently associated with MMC treatment. Other adverse effects include punctal stenosis, conjunctivitis, photosensitivity and allergic reaction.
Because of the risk of punctalcanalicular stenosis, it is important to place punctal plugs to prevent drainage into the nasolacrimal ducts. Another benefit of punctal occlusion is that it lengthens contact time between the medicine and the ocular surface.
| 5-FU. The antimetabolite 5-FU blocks thymidine synthase, affecting the ability of cells to make nucleic acids and, therefore, necessary proteins. Tumor cells are preferentially affected because of their rapid doubling time. |
Treatment of OSSN with topical 5-FU has been effective, with an excellent clinical response (Figs. 2A and 2B) and a 7.3 percent recurrence rate in one series.3 A typical regimen of 1 percent topical 5-FU is four drops daily for either four to seven days on/30 days off or continuously for three to four weeks. The cycling regimen seems to be as efficacious as continuous application and exposes the patient to less toxicity.
Although the adverse effects of 5-FU are less severe than those of MMC, they include corneal epitheliopathy, conjunctival inflammation and irritation. Some experts suggest manual punctal occlusion for a minute after drop instillation.
IFN-a2b. This low-molecularweight glycoprotein, produced by leukocytes, has antineoplastic and antiviral properties. It works through a number of mechanisms, including slowing the cellular growth cycle and promoting the body’s immune and antitumor response. The FDA has approved IFN-a2b for the treatment of several conditions, including hairy cell leukemia.
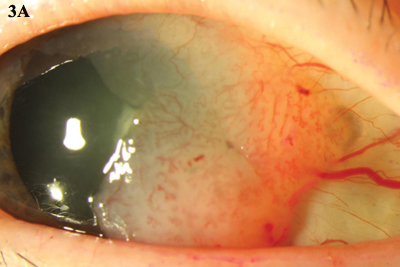
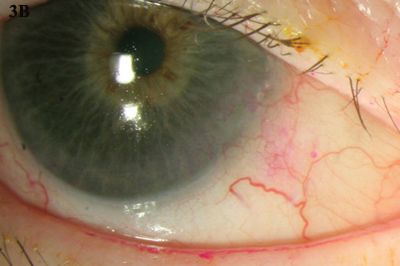
IFN-a2b can be administered via topical drops (Figs. 3A and 3B) or subconjunctival injections. With drops, clinical resolution usually takes place with a mean treatment time of about 12 weeks. Subconjunctival injection combined with topical IFN-a2b for noninvasive OSSN has a faster time to resolution, about six weeks.4 In one study, the overall response rate was 96.4 percent, and the recurrence rate was 3.7 percent after one year.5
A typical regimen consists of topical IFN-a2b drops with a concentration of 1 million IU/mL or 3 million IU/mL, applied four times daily; or subconjunctival injections of 3 million IU/0.5 mL, administered weekly. No significant difference has been demonstrated.
When given topically, IFN-a2b drops are generally well tolerated and have minimal side effects. Some mild systemic effects, such as fatigue, myalgia and fever, have been described with subconjunctival injections. These side effects usually can be treated effectively with acetaminophen.
While topical IFN-a2b drops are gentle to the ocular surface, some patients will need to use the drops continually for 12 weeks or more; thus, compliance must be emphasized. No punctal plugs are needed.
|
|
|
INTERFERON. (3A) Topical IFN-a2b was used to treat this gelatinous CIN. (3B) The lesion resolved over a five-month period.
|
Summary
Surgical excision with wide margins followed by cryotherapy has been the gold standard for the treatment of OSSN. However, topical chemotherapy is emerging as an effective alternative to surgery and is gaining in popularity as a primary treatment, particularly for recurrent, annular or larger lesions. Topical chemotherapy also may be used preoperatively, for chemoreduction, or postoperatively, for treating positive margins or recurrent disease.
Although there have been no randomized, controlled trials of the drugs MMC, 5-FU and IFN-a2b for treatment of OSSN, each has been reported to be effective. In deciding which agent to select, the clinician should weigh multiple factors, including possible side effects, cost and compliance.
Generally, one should begin to see a clinical response after a month (or after one to two cycles) of treatment. If no improvement is observed after this amount of time, another drug or surgery should be considered.
___________________________
1 Tabin G et al. Ophthalmology. 1997;104(3):485-492.
2 Russell HC et al. Br J Ophthalmol. 2010;94(10):1316-1321.
3 Parrozzani R et al. Br J Ophthalmol. 2011;95(3):355-359.
4 Karp CL et al. Ophthalmology. 2010;117(12):2241-2246.
5 Schechter BA et al. Ophthalmology. 2008;115(8):1291-1296.
___________________________
Mr. Jaber is a medical student at Dartmouth Medical School in Hanover, N.H. Dr. Karp is professor of clinical ophthalmology at Bascom Palmer Eye Institute in Miami. The authors report no related financial interests.
At a Glance: Treatment Options
| |
MMC |
5-FU |
IFN-a2b |
Dose and
schedule |
0.02 percent or
0.04 percent MMC,
4 times daily.
1-2 weeks on;
1-2 weeks off. |
1 percent topical
5-FU, 4 times daily.
4-7 days on; 30
days off—or con-
tinuously for 3-4 weeks. |
Topical: 1 million
IU/mL IFN-a2b,
4 times daily.
Subconjunctival:
3 million IU/0.5 mL;
0.5 mL weekly. |
| Refrigeration |
Yes |
No |
Yes |
| Cost |
About $200/cycle |
About $80/cycle |
About $200/month |
| Side effects |
Substantial: pain;
punctal stenosis;
epitheliopathy. |
Moderate: conjunctival
irritation; pain;
epitheliopathy. |
Mild: drops well
tolerated; injections
may give transient
flu-like syndrome. |
|