Neurotrophic Keratitis: Topical rhNGF Is Safe and Effective
By Lynda Seminara
Selected By: Stephen D. McLeod, MD
Journal Highlights
Ophthalmology, September 2018
Download PDF
Bonini et al. assessed the safety and efficacy of topical recombinant human nerve growth factor (rhNGF) for treatment of moderate to severe neurotrophic keratitis (NK). Their results affirmed earlier safety findings and demonstrated the healing and neuroprotective effects of rhNGF in patients with this disease.
This multicenter phase 2 study was vehicle controlled, randomized, and double masked.
Patients with moderate (stage 2) or severe (stage 3) NK in 1 eye were eligible to participate. Those who met all inclusion criteria (N = 156) were assigned randomly (1:1:1) to receive rhNGF 10 μg/mL, rhNGF 20 μg/mL, or vehicle. The dosage for each study arm was 6 drops per day for 8 weeks. Follow-up ensued for at least 48 weeks. The main measure of efficacy was corneal healing, defined as total lesion-area diameter <0.5 mm by fluorescein staining. Assessments were made by centralized masked readers at week 4 of follow- up (primary endpoint) and again at week 8 (key secondary endpoint). Corneal healing also was assessed post hoc, using a more conservative measure (0-mm stained lesion area and no other visible staining). The primary variable for safety assessment was the incidence of adverse events.
By the 4-week follow-up, corneal healing had occurred in 54.9% of the 10-μg/mL rhNGF group, 58% of the 20-μg/mL rhNGF group, and 19.6% of the vehicle-control group. By week 8, the respective rates of corneal healing were 74.5%, 74%, and 43.1%, respectively.
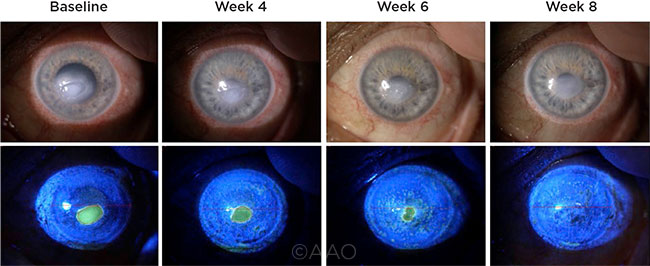 |
KERATITIS TREATMENT. This patient—who had an oval, acentral, neurotrophic corneal lesion—was treated with 20 μg/ml rhNGF. Photographs taken from baseline through week 8 under diffuse white light (top row) and cobalt-blue light (bottom row) illumination.
|
Post hoc analysis by the more conservative measure also demonstrated significant differences, at both time points, between active treatment and vehicle control. More than 96% of patients whose cornea healed from rhNGF treatment remained free of recurrence throughout follow-up.
Few patients experienced adverse events (AEs), most of which were local, mild, and transient, and did not require cessation of treatment. The highest incidence of AEs was in the control group.
Findings of this study indicate that the benefits of rhNGF outweighed the risks for patients with moderate to severe NK.
The rhNGF treatment also holds promise for other ophthalmic conditions with neurodegenerative components, such as glaucoma, macular degeneration, and retinitis pigmentosa.
The original article can be found here.