By Jennifer S. Griffin, MS, Contributing Writer, interviewing Joseph Caprioli, MD, Catherine Green, FRANZCO, and Joel Schuman, MD
Download PDF
Ocular hypotony is a relatively common consequence of trabeculectomy, but is it always a problem? The reality is that an intraocular pressure (IOP) that is harmfully low in one patient can be innocuous, or even beneficial, in another.
Joseph Caprioli, MD, at the University of California, Los Angeles, said, “In some patients, detrimental effects of hypotony, such as maculopathy, occur at a pressure of 8 or 10 mm Hg. Others can have a pressure of 2 mm Hg and the eye functions well. I wouldn’t consider these latter patients to be physiologically hypotonous, and a pressure of 2 mm Hg is probably very good for their glaucoma.” Joel Schuman, MD, at the New York University School of Medicine, noted, “It depends on the patient as to whether the eye can withstand a certain pressure, be it too high or too low.”
When it comes to managing patients with trabeculectomy-induced hypotony, some questions to consider include the following: How frequently should you monitor? When should you intervene? And which surgical procedures yield the best results?
 |
|
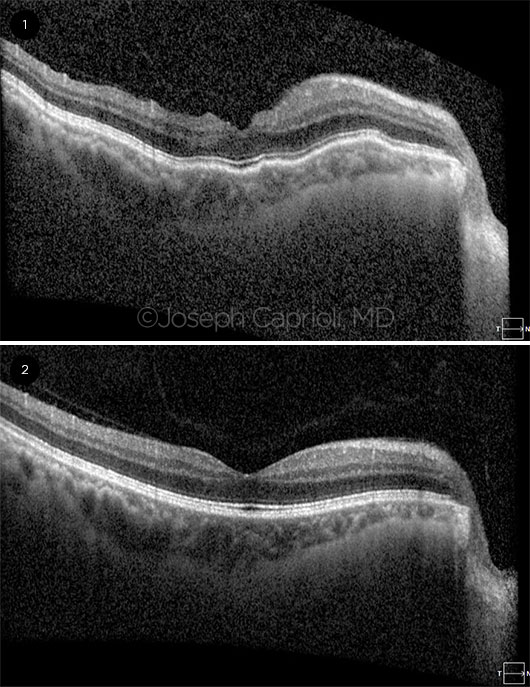
POSTOP VIEWS. Signs of hypotony maculopathy depicted by optical coherence tomography (1) after primary trabeculectomy and (2) following subsequent bleb revision to raise IOP. The images were obtained 10 months apart.
|
Hypotony and Its Pathophysiology
Hypotony has commonly been considered to be an IOP of 6.5 mm Hg or less, which is >3 standard deviations below the mean for nonglaucomatous eyes.1 The use of antifibrotic agents such as mitomycin C (MMC) during trabeculectomy has coincided with an increase in rates of postsurgical hypotony.2 Recent reports indicate that the rate of hypotony after trabeculectomy is variable and difficult to predict, ranging from slightly less than 2%3 to more than 40%.4
Pathophysiology. In practice, the numeric definition of hypotony does not necessarily correspond to poor outcomes.3 Dr. Schuman said, “If the patient is tolerating the low pressure, hypotony in and of itself is not a reason to intervene.” In a retrospective case-control study, Tseng et al.3 determined that patients who experienced hypotony after trabeculectomy (defined as IOP ≤5 mm Hg at ≥3 months postoperatively) shared similarities with those who did not, including reoperation rate, vision loss, and bleb-related infection. Dr. Caprioli, who coauthored that study, said he prefers the following pathophysiologic definition of hypotony: “the pressure that causes secondary changes in the eye, such as swelling in the retina, swelling of the optic disc, folds in the choroid, and folds and swelling of the cornea.” These secondary changes signal that the patient has hypotony maculopathy, a worrisome complication of hypotony.
Hypotony maculopathy. Hypotony maculopathy involves chorioretinal wrinkling, papilledema, vascular tortuosity, and vision loss.5 If left untreated, these structural and functional effects can become irreversible. To correct hypotony maculopathy, the IOP must be raised, which presents a challenge to glaucoma surgeons. “You want to raise the pressure so that it causes the maculopathy to resolve but does not add to the risk of glaucomatous progression,” said Dr. Caprioli.
Target Setting
The experts advocate setting a target IOP value before surgery, based on the patient’s condition and the extent of glaucomatous damage. “For all patients undergoing trabeculectomy, I aim for a low IOP—as close to 10 mm Hg as possible, lower if needed,” said Catherine Green, FRANZCO, at the Royal Victorian Eye and Ear Hospital in East Melbourne, Australia.
Dr. Schuman shared his basic guidelines for setting a target IOP. “For patients with severe glaucoma, I might want a pressure of 12 mm Hg or less. If they have moderate glaucoma, 15 to 16 mm Hg might be okay. For early glaucoma, the target IOP might be in the high teens or low 20s.”
Warning Signs
After trabeculectomy, frequent monitoring is important. In her practice, Dr. Green follows up with patients on “days 1 and 5 and weekly for the first 4 to 6 weeks—more frequently if there are concerns.”
Early resolution. In the early postsurgical period, hypotony may be accompanied by “macular edema and choroidal folds associated with decreased vision, but these consequences often resolve spontaneously,” said Dr. Caprioli. However, if they don’t resolve, waiting too long to intervene can result in permanent damage. “I usually take action if these consequences of hypotony still are present at 3 or 4 months,” he said.
Vision changes. Dr. Caprioli stressed that “regardless of the pressure, the vision has to be monitored very carefully postoperatively. If the visual acuity drops for no other apparent reason,” he continued, “it’s important to examine the fundus for swelling of the retina, folds in the choroid, or swelling of the optic disc. On slit-lamp exam, you can see if there are changes in the cornea; but typically, changes in the retina are more sensitive to low pressure and precede changes in the cornea.” Dr. Schuman stated, “If a person has hypotony maculopathy, I’m going to be aggressive in the intervention because there is a limited time window in which you can restore excellent visual acuity.”
Preferred Treatments
In some cases, consequences of hypotony can be managed nonsurgically in the early postoperative period “with atropine and by reducing steroids to try to promote fibrosis,” said Dr. Green. However, hypotony maculopathy requires surgical correction.
Revisions. Dr. Green listed her preferences: “transconjunctival suture of the flap, revision of the bleb, or resuturing of the flap.” She added, “For the latter, I always have sclera available in case a scleral patch graft is needed.” She cautioned that after trabeculectomy, “the sclera can be friable and difficult to handle, especially if MMC has been used.” If flow through the ostium cannot be reduced, she recommends applying a patch graft.
Dr. Schuman detailed his process: “You look for a leak, a cyclodialysis cleft, or inflammation to indicate why the pressure is so low. If there’s an excellent bleb, then the solution may be limiting the bleb to raise the pressure while staying within the therapeutic window. The first thing I try is compression sutures. If someone has hypotony maculopathy, you might need to be even more aggressive than that.” Dr. Schuman noted that, in a case study of recalcitrant hypotony,6 he and a colleague found that “instillation of perfluorocarbon liquid was successful” in mechanically flattening the retina and choroid and improving vision.
Dr. Caprioli advised, “If the flap is visible, it is often possible to tighten the scleral flap by placing an extra suture transconjunctivally. If you can’t see the flap, then the bleb has to be taken down surgically, and the flap has to be tightened directly by adding an extra suture or with tissue patching.” In a retrospective series of 33 patients with post-trabeculectomy hypotony maculopathy, Dr. Caprioli and colleagues found that surgical revision of the bleb improved best-corrected visual acuity long term in 29 (88%) of them.7
Tips for Trabeculectomy
Can a patient’s risk for problematic trabeculectomy-induced hypotony be predicted, and can this outcome be avoided? “Surgeons who perform trabeculectomy generally agree on several risk factors for hypotony maculopathy: younger age, myopia, and a history of vitrectomy,” explained Dr. Caprioli. “These factors all are related to the rigidity of the sclera. If the sclera is very compliant and soft, it’s more likely to collapse and fold at a lower pressure, which we think is probably one of the causes of hypotony maculopathy.” He added, “What we teach—based on collective experience—is that if you have a young myopic male, he is at higher risk for hypotony maculopathy at relatively higher pressures.”
Avoid leaks. Dr. Green and her colleagues recently completed an International Council of Ophthalmology Surgical Competency Assessment Rubric (ICO-OSCAR) in trabeculectomy.8 She said that “good conjunctival closure is essential to prevent wound leak, which would likely cause early hypotony and would require much earlier intervention.” Dr. Green explained, “Placement of flap sutures is what determines aqueous flow and is the main determinant of hypotony risk. Sutures need to be tight enough to result in minimal flow at time of surgery.”
Titrate the flow. Dr. Caprioli shared his tips for achieving a low target IOP in trabeculectomy: “I place extra sutures or sutures that are a little tight to start with and may yield an IOP that’s a little higher than the target. Then in the first few weeks, I selectively cut the sutures in the office with a laser to release tension on the flap and let more fluid through, thereby lowering the IOP to target.”
Manage expectations. In certain subgroups of patients, such as those with uveitic glaucoma, detrimental hypotony can be common. Dr. Green and colleagues recently conducted a review of uveitic glaucoma management9 and found hypotony to be the most common complication of trabeculectomy or glaucoma device implantation, affecting approximately 30% of patients. She pointed out, “In patients with active uveitis, there is a higher risk of bleb failure, so there is a tendency to use a higher dose of MMC, but this may increase the risk of hypotony.”
For particularly high-risk patients, Dr. Green emphasized the importance of preoperative discussions with the patient as part of the consent process: “Managing hypotony can be challenging for both surgeon and patient, so having this understood makes the process slightly easier if it does occur.”
___________________________
More online. Ruth D. Williams, MD, interviews Anne Louise Coleman, MD, PhD, about ocular hypotony at aao.org/interview/ocular-hypotony.
___________________________
1 Pederson JE. Ocular hypotony. In: Ritch R, Krupin T, Shields MB, eds. The Glaucomas. 2nd ed. St. Louis: Mosby; 1996:385-395.
2 Costa VP et al. Ophthalmic Surg. 1993;24(6):389-394.
3 Tseng VL et al. Ophthalmology. 2017;124(10):1457-1465.
4 Bindlish R et al. Ophthalmology. 2002;109(7):1336-1341.
5 Costa VP, Arcieri ES. Acta Ophthalmol Scand. 2007;85(6):586-597.
6 Duker JS, Schuman JS. Ophthalmic Surg. 1994;25(7):463-465.
7 Bitrian E et al. Am J Ophthalmol. 2014;158(3):597-604.
8 Green CM et al. J Glaucoma. 2017;26(9):805-809.
9 Kwon HJ et al. Clin Exp Ophthalmol. 2017; 45(5):472-480.
___________________________
Dr. Caprioli is an ophthalmologist at the Ronald Reagan UCLA Medical Center and is professor of ophthalmology, University of California, Los Angeles. Financial disclosures: None.
Dr. Green is an ophthalmologist and Head of the Glaucoma Unit, Royal Victorian Eye and Ear Hospital, East Melbourne, Australia. Financial disclosures: Allergan: C,L.
Dr. Schuman is an ophthalmologist at NYU Eye Center, NYU Langone Health, and is a professor of ophthalmology at New York University School of Medicine. Financial disclosures: Aerie Pharmaceuticals: C; BrightFocus Foundation: S; Carl Zeiss Meditec: P; Department of Defense: S; National Eye Institute: S; Ocugenix: O,P; Ocular Therapeutix: C,S; Opticient: C,O; Slack: C.
See the disclosure key at www.aao.org/eyenet/disclosures.