By Linda Roach, Contributing Writer, interviewing Justis P. Ehlers, MD, Prithvi Mruthyunjaya, MD, MHS, and Scott D. Schoenberger, MD
Download PDF
Abundant level I clinical trial evidence shows that intravitreal pharmacotherapy can safely and effectively treat macular edema secondary to branch retinal vein occlusion, with better visual outcomes than laser photocoagulation, an Academy Ophthalmic Technology Assessment (OTA) panel has concluded.
Assessing the Evidence
In a study published by Ophthalmology,1 the OTA Committee Retina/Vitreous Panel reported finding high-quality evidence from 16 well-designed studies supporting the use of intravitreal therapy with steroids or anti–vascular endothelial growth factor (VEGF) drugs to manage BRVO.
“There’s clear evidence that both anti-VEGF agents and steroids show great benefits in terms of both anatomic response and visual acuity, particularly for those patients who have significant vision loss caused by macular edema,” said Justis P. Ehlers, MD, at the Cleveland Clinic, in Cleveland, Ohio.
“For quite some time, the focus of our therapy in branch retinal vein occlusion was laser photocoagulation. Now, pharmacotherapy has really become first-line therapy for managing this condition,” Dr. Ehlers said.
Ramping up the research. “One of the things that stood out in our literature review was the amount of research that has been done in this area,” Dr. Ehlers said. “The overall number of manuscripts related to the management of branch retinal vein occlusions was really quite large.”
 |
|
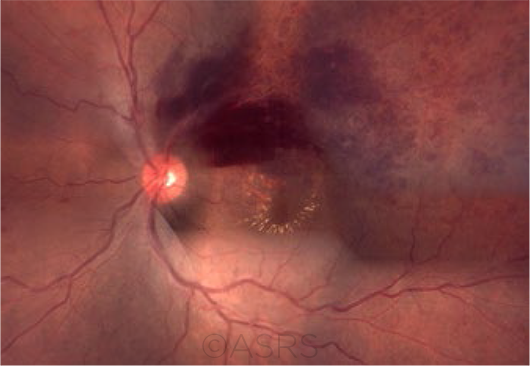
THREAT TO VISION. Clinical features of BRVO may include sectoral retinal hemorrhages, dilated and tortuous retinal vessels, and cotton-wool spots in the distribution of the occluded vein. This patient had macular edema following BRVO; the fundus photograph composition shows flame-shaped and blot hemorrhages in the superotemporal quadrant, with hard exudates surrounding the fovea. This image was originally published in the ASRS Retina Image Bank. Gerardo Garcia-Aguirre, MD. Branch Retinal Vein Occlusion With Macular Edema. Retina Image Bank. 2012; Image Number 322. © The American Society of Retina Specialists.
|
Moving Away From Using Laser
Macular grid laser photocoagulation became the gold standard for BRVO treatment after the publication in 1984 of results from the Branch Vein Occlusion Study (BVOS).2 Indeed, as recently as 2015, a review of the literature available through August 2014 concluded that the evidence was insufficient to support using intravitreal pharmacotherapy instead of laser for primary treatment of BRVO.3
But the OTA panel’s report, which reviewed trials reported through January 2017, disagrees. “Multiple pharmacotherapies appear to be safe and effective treatments for macular edema secondary to BRVO. Level I evidence has shown anti-VEGF therapy, in particular, to result in significant visual acuity improvement compared with laser or observation,” the authors wrote.
Resolution of edema. Clinicians have moved away from laser therapy for BRVO over the last decade because of reports of faster resolution of the edema, as measured by optical coherence tomography (OCT), and better visual outcomes after intravitreal pharmacotherapy, said Prithvi Mruthyunjaya, MD, MHS, at the Byers Eye Institute in Palo Alto, California. The OTA analysis confirmed this, he said. “With laser, you just don’t get the same response in terms of how quickly the edema goes away and how quickly you see the visual improvement.”
From BVOS to today. The earlier BVOS results were limited by the lack of imaging technology—such as OCT—that today’s researchers can use to measure therapeutic effects, Dr. Mruthyunjaya said.
Furthermore, the BVOS defined treatment success less stringently than now is the case. The study reported on the number of patients who, at 3 years, were seeing 2 or more lines (10 letters) better than when treatment began (65% of eyes treated with laser, versus 37% in control eyes, who were observed only). Today’s trials look for 3 lines (15 letters) of acuity improvement after therapy.
Nuances of patient selection. Nonetheless, the OTA panel did not take a stand against laser photocoagulation, Dr. Ehlers said. “There may be select patients who because of their case characteristics may still be good candidates for laser,” he said. “In addition, initial observation may be considered, particularly for patients with mild macular edema and minimal vision loss.”
Anti-VEGF Tx: Strong Benefits
Level I evidence cited in the OTA shows that delays in initiating anti-VEGF injections can result in irreversible vision loss, said Scott D. Schoenberger, MD, who practices in Dayton, Ohio. “If treatment is delayed 6 months, the patient does not regain as much vision as if they were treated promptly,” he said.
Looking to BRAVO results. For instance, in BRAVO,4 the first large randomized, controlled trial to look at VEGF inhibition to treat BRVO, researchers compared outcomes at 1 year with 2 different doses (0.3 mg and 0.5 mg) of ranibizumab to a delayed-treatment control group.5 (After 6 months, patients in this group were switched to ranibizumab for 6 months.)
Visual acuity. The percentage of patients who gained 15 or more letters in best-corrected visual acuity (BCVA) was 55.2% for those who received 0.3 mg of ranibizumab and 61.1% for those who received 0.5 mg, compared with 28.8% in the sham group.
Foveal thickness. Central foveal thickness decreased by 337 μm and 345 μm in those who received 0.3 mg and 0.5 mg ranibizumab, respectively, and by 158 μm in the sham group.
Lagging behind. Although the crossover group received ranibizumab injections after 6 months of sham injections, their vision did not recover to the same level as the other groups. At the 12-month mark, the treated groups had improved by 16.4 (14.5-18.4) letters in the 0.3-mg group and 18.3 (15.8-20.9) letters in the 0.5-mg group, compared to 12.1 (9.6-14.6) letters in the sham crossover group (p < 0.01 for each dose group versus sham).
Don’t delay treatment. The results seen with the crossover group in BRAVO point to the need to begin anti-VEGF therapy in a timely manner. “That’s one of the takeaways from our report,” Dr. Ehlers said. “If you have a patient who presents with more severe vision loss and macular edema, you probably [will] want to minimize any significant delays in initiating therapy.”
Steroids: Pros and Cons
Six well-designed, controlled clinical trials yielded level I evidence supporting the use of intravitreal steroids in BRVO, the panel reported. However, those studies showed that, unlike VEGF inhibitors, steroids come with the risks of cataract progression and a rise in intraocular pressure. These ocular side effects are fewer with the Ozurdex intravitreal implant (0.7 mg dexamethasone) than with intravitreal injections of triamcinolone, the evidence showed.
“A lot of people consider steroids to be a second-line pharmacological treatment because of the risks,” Dr. Schoenberger said. “But some people don’t respond to anti-VEGF therapy, and for that reason steroids definitely have a role in treatment.”
In its summary, the OTA panel wrote: “Corticosteroid trials have exhibited favorable results. Dexamethasone implant trials have shown more rapid improvement in visual acuity compared with sham injections and intravitreal triamcinolone, demonstrating similar results to grid laser photocoagulation, but intravitreal corticosteroids are associated with more frequent ocular side effects.”
In the Clinic
The OTA panel envisions its report as being helpful to comprehensive ophthalmologists who see patients with BRVO in their practices and who might decide to manage the disease without referral, Dr. Mruthyunjaya said. The OTA report also can be helpful for ophthalmologists in counseling their patients who develop BRVO, he said.
“We know that diseases like branch retinal vein occlusion are managed not only by retina specialists but also by comprehensive ophthalmologists and even other specialists. So this type of information can help guide them in terms of how to evaluate the data on the available treatments,” he said.
Dr. Ehlers also said that he hopes the OTA will alert comprehensive ophthalmologists as to how far the field has come since the days when laser photocoagulation was the only treatment option for macular edema from BRVO.
“It’s important for them to know that we have a lot of treatment options that have been clearly established in high-level clinical trials to be effective in the management of branch retinal vein occlusion—and because of that, it’s definitely worthwhile to consider early referral,” he said. “The data are clear that significant delays in therapy may limit overall response to therapy.”
___________________________
1 Ehlers JP et al. Ophthalmology. Published online May 24, 2017.
2 The Branch Vein Occlusion Study Group. Am J Ophthalmol. 1984;98(3):271-282.
3 Lam FC et al. Cochrane Database Syst Rev. 2015 May 11;(5):CD008732.
4 BRAVO = ranibizumaB for the treatment of macular edema following bRAnch retinal Vein Occlusion
5 Campochiaro PA et al. Ophthalmology. 2010:117(6):1102-1112.
___________________________
Dr. Ehlers holds the Norman C. and Donna L. Harbert Endowed Chair for Ophthalmic Research at the Cole Eye Institute of the Cleveland Clinic in Cleveland, Ohio. He is also a member of the Academy’s OTA Committee Retina/Vitreous Panel. Relevant financial disclosures: Genentech: S; Regeneron: S.
Dr. Mruthyunjaya is director of ocular oncology and associate professor of ophthalmology, ocular oncology, and vitreoretinal surgery at the Byers Eye Institute at Stanford University in Palo Alto, Calif. He is also a member of the Academy’s OTA Committee Retina/Vitreous Panel. Relevant financial disclosures: None.
Dr. Schoenberger is a vitreoretinal specialist and partner at Retina Physicians & Surgeons in Dayton, Ohio. He is also a member of the Academy’s OTA Committee Retina/Vitreous Panel. Relevant financial disclosures: None.
For full disclosures and the disclosure key, see below.