Download PDF
With medication and laser on one end and trabeculectomy and tube shunts on the other, MIGS is poised to occupy the middle ground. But which patients are the best candidates? Here’s an overview of surgical pearls and a look at some key considerations.
There’s no question that MIGS is one of the hot areas in glaucoma today. Whether you define MIGS as minimally invasive or microinvasive glaucoma surgery, the procedures are intended to improve outflow either by bypassing (or eliminating) the trabecular meshwork or by shunting aqueous humor into the suprachoroidal or subconjunctival space.
Currently, only two MIGS devices are commercially available—the iStent (Glaukos) and the Trabectome (NeoMedix), although others are in development (see “Looking Ahead: In the Pipeline”). Here’s an overview of how to perform surgery with these devices, plus a look at some significant cautions and concerns.
 |
|
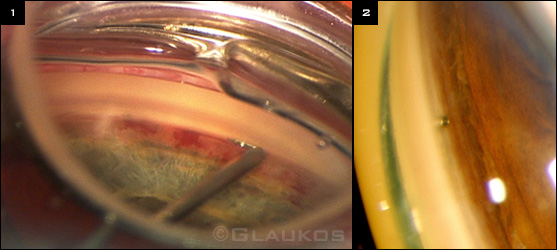
iSTENT. (1) During implantation. (2) Gonioscopic view of the stent in Schlemm’s canal.
|
Before You Begin
All definitions of MIGS “would include the fact that a MIGS procedure is far less tissue disruptive than the more traditional glaucoma surgeries,” said Thomas W. Samuelson, MD, at Minnesota Eye Consultants in Minneapolis. “The primary intent is to have a safer, less invasive option for patients with glaucoma as compared to trabeculectomy and tube shunts.”
NEED TO READJUST TECHNIQUE. MIGS surgery involves a “significant learning curve,” as techniques and visualization are considerably different from traditional glaucoma and cataract surgery, said Kevin Kaplowitz, MD, at Stony Brook University in Stony Brook, N.Y.
Key steps in all MIGS procedures include properly identifying the trabecular meshwork, the landmark seen on gonioscopy; avoiding undue outward pressure; and confirming proper placement of devices or ablation instruments.
In some respects, surgery with iStent and Trabectome are the same, said Brian A. Francis, MD, MS, at the Doheny Eye Institute in Los Angeles. “The only difference is, once you get into the area with the device, you either implant a stent or ablate tissue with the handpiece.”
But “getting into the area” requires a total readjustment of surgical technique. In MIGS surgery, the patient, the microscope, and even the surgeon’s hands are positioned differently than in cataract surgery.
NEED FOR GONIOSCOPY. Dr. Samuelson urged physicians to practice intraoperative gonioscopy before even beginning to work with MIGS. He recommended that surgeons take the following steps:
- Turn the patient’s head 45 degrees away from you;
- rotate the microscope head 45 degrees toward you (that 90 degrees allows you to view the angle);
- put a coupling gel on the cornea; and
- gently touch the meshwork and get used to the feel and ergonomics of the maneuver.
 |
|
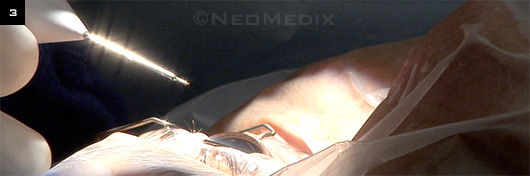
TRABECTOME. Handpiece poised to enter the anterior chamber. Note head tilt, which is accompanied by a tilt of the microscope totaling 60 to 70 degrees.
|
Using the iStent
The iStent, approved in 2012, is designed to increase outflow by directly bypassing the inner wall of Schlemm’s canal. The stent, which is preloaded into an injector, is inserted in the canal through a 1.5-mm corneal incision. “It offers access to Schlemm’s canal with very little tissue disruption,” Dr. Samuelson said. The learning curve includes getting a sense of the trabecular meshwork. “It’s a very distinctive feel when you enter the canal and know the stent is in the proper place,” Dr. Samuelson said. He added that angle landmarks will be familiar to the glaucoma surgeon. “If they’ve done goniotomy, they’ll know the feel of the trabecular meshwork,” he added.
PATIENT SELECTION. For their first cases, surgeons should select patients who are right on the margin—that is, those who could get by with cataract surgery alone but who might possibly get additional benefit of a “plus MIGS” procedure, Dr. Samuelson said. “If the procedure doesn’t go as well as hoped, or if for some reason the stent can’t be implanted, it helps to know that the patient will do fine with cataract surgery alone but will likely require more postoperative medication.”
Dr. Samuelson has started to push the envelope and use the iStent in highly selected, more-advanced cases by going off label and using more than one stent. He said doctors often ask if they should wait to adopt iStent until two stents are approved. “I would strongly encourage mastering one stent before considering two,” he said. “The learning curve for a single stent placement is challenging enough for initial cases.”
SURGICAL PEARLS. Dr. Samuelson offered the following pointers.
- He prefers inserting the stent after cataract surgery, but this step can be done before or after phaco.
- If you prefer right eyes for cataract surgery, you may also feel more comfortable performing your first few stent cases in right eyes. Also, if you prefer the forehand maneuver of the “left” iStent, it is acceptable to use the left stent in either right or left eyes. Likewise, it is acceptable to use “right” (backhand maneuver for right-handed surgeons) stents in either left or right eyes. The stents are interchangeable, so once you get the feel for the surgery, you may migrate to one stent type or the other (i.e., “left” or “right” stents).
- Turn the microscope toward you and the patient’s head away from you. It’s important to approach the canal at an angle.
- Maintain adequate anterior chamber stability with an ophthalmic viscoelastic device (OVD) and a very light touch with the gonioprism.
- Once the tip of the iStent pierces the meshwork, it’s important to lower the heel of the stent and raise the toe to slide it into the canal.
- Make sure all of the OVD is evacuated from the anterior chamber and from behind the IOL.
POSTOPERATIVE CARE. It takes at least six weeks to reach a new steady-state baseline pressure. Because patients are vulnerable to a steroid response, Dr. Samuelson tapers steroids more rapidly than normal: four times daily for the first week; twice daily for the second week; then stop.
Don’t be overzealous in stopping glaucoma medications. Dr. Samuelson’s average iStent patient is on 2.3 medications before surgery. For patients on two or more medications, he continues at least one in most instances but may stop all meds in lower-risk eyes. If the patient is on just one medication, he’ll generally discontinue it. “It’s okay to stop one or two, but be cautious about stopping three or four.”
Pearls From the Experts
DR. CAPRIOLI: “For patients who have optic nerve damage and visual fields that are getting worse, the mild efficacy achieved with MIGS is not, in the majority of cases, the answer.”
DR. FRANCIS: “You want to pick patients who require pressure lowering, but not to an extremely low level, and for whom you want to reduce the number of medications. For a standalone procedure [without cataract surgery], my typical patient is somebody who has a high IOP, generally in the 20s, and is uncontrolled on generally two or more medications.”
DR. GEDDE: “Consideration may be given to MIGS in combination with cataract surgery in patients with mild to moderate glaucoma, especially if they’re having difficulty with medical therapy. Traditional glaucoma surgery is generally preferred in patients with advanced glaucoma or progressive disease in which low levels of IOP are needed.”
DR. KAPLOWITZ: “With these devices, it is vital to resist the natural tendency to continue pushing the instrument tip toward the sclera, which can damage Schlemm’s canal.”
DR. SAMUELSON: “You might be surprised at how delicate the trabecular meshwork is. The process of implanting the iStent within the canal and releasing it is about as delicate a maneuver as we encounter in anterior segment surgery.” |
Using the Trabectome
The Trabectome, which was approved in 2004, uses electrocautery to ablate and permanently remove a portion of the trabecular meshwork and inner layer of Schlemm’s canal within the anterior chamber to allow for increased aqueous outflow.1 “I like the fact that Trabectome targets the outflow system,” Dr. Francis said.
The Trabectome combines well with small incisions and with clear corneal cataract surgery, but it can be done as a standalone procedure, he added. “It’s a very visual procedure. There’s no tactile feedback. The key is visualization of the angle structures. You have to treat the right place. But once you get visualization, it’s not a hard surgery to do.”
 |
|
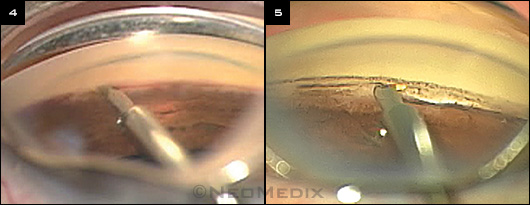
TRABECTOME. (4) Electrocautery is applied with a proprietary handpiece to ablate a portion of the trabecular meshwork. (5) Trabectome inside eye.
|
PATIENT SELECTION. Although usage indications have been expanding, the Trabectome is intended for primary and secondary open-angle glaucoma, Dr. Francis said. “Some of the best results we see are in the secondary glaucomas, such as pseudoexfoliation or pigmentary glaucoma.”
But typically, no matter how high the starting pressure, the reduction tends to reach the same level. Whether the starting pressure is 40 mmHg or 17 mmHg, the final pressure will be 15 or 16 mmHg. “If the patient requires a pressure of 10, I wouldn’t do Trabectome,” Dr. Francis said. But if they’re at 17 mmHg and you want to reduce the number of medications, you may consider a MIGS procedure, he added.
Dr. Samuelson noted that patients with trabeculodysgenesis (a juvenile-onset glaucoma with an inherent abnormality of the meshwork) might benefit from removal of several clock hours of meshwork using the Trabectome.
SURGICAL PEARLS. Using the corneal incision site as a fulcrum, slowly advance the instrument along the meshwork in clockwise or counterclockwise direction up to the limit of good visualization. This usually creates an arc of 90 to 120 degrees ablated.
Key to success is identification of the trabecular meshwork, Dr. Francis said. This can be done by identifying the pigment in the angle. If the angle has no pigment, or if Dr. Francis is confused about landmarks, he induces hypotony at the start of surgery by releasing the aqueous and letting blood reflux and pool in Schlemm’s canal. “The target is where the blood is,” he said. “Go straight at that with your Trabectome.”
POSTOPERATIVE CARE. This varies by surgeon. Dr. Francis discharges his patients on the same day, with a light dressing or shield applied to the operative eye. His postoperative routine includes the following:
- Topical antibiotic drops are given four times daily for seven days.
- Steroid drops, four times daily, are tapered over eight weeks.
- Pilocarpine (1 or 2 percent) is used in the operative eye two to four times daily, tapered over two to eight weeks. Pilocarpine is intended to minimize formation of peripheral anterior synechiae and to help with short-term IOP reduction.
- All preoperative glaucoma medications may be restarted immediately and tapered the first postoperative day, depending on IOP.
Looking Ahead: In the Pipeline
The search for a better MIGS device continues. Here’s a look at some devices in the pipeline, grouped according to the reservoir that receives the aqueous humor.
Canal-based approach. Like the iStent, the Hydrus Microstent (Ivantis) is inserted ab interno into Schlemm’s canal and is intended to improve flow from the anterior chamber into the canal. But it is 8 mm long, compared with the 1.0 mm iStent. Dr. Samuelson said it “also slightly distends the canal, which in a laboratory setting further enhances outflow.”
Suprachoroidal-based procedures. Using an ab interno approach, these devices are inserted through the angle and completely bypass the meshwork to connect the anterior chamber with the suprachoroidal space. Two in development are the CyPass Micro-Stent (Transcend Medical) and the iStent Supra (Glaukos).
Subconjunctival-based options. The AqueSys XEN (AqueSys) involves a translimbal stent that is inserted using an ab interno transscleral approach, connecting the anterior chamber to the subconjunctival space. The InnFocus MicroShunt (InnFocus) connects the anterior chamber to the subconjunctival space using an ab externo trans-scleral approach.
Which approach is best? Ultimately, the indications for the devices are similar and overlap, Dr. Kaplowitz said. “I have not yet seen any literature suggesting that there are particular indications for using one device over the other.” Thus, at this time, the choice of device would rest on the surgeon’s personal preference. |
Current Concerns
On one hand, there is general agreement that MIGS devices have a better safety profile than tubes or trabeculectomy. On the other, MIGS procedures are also less effective at lowering pressure. “For all the devices, patients should have an IOP goal in the midteens, as lower pressures cannot be relied on,” said Dr. Kaplowitz, lead author on a review of MIGS techniques and outcomes.2
NOT A PERFECT SOLUTION. Joseph Caprioli, MD, at the Jules Stein Eye Institute in Los Angeles, agreed that the two approved MIGS procedures are safer than trabeculectomy in terms of major side effects. “But the treatment effects are small. MIGS is not a panacea.” He compared the effect to adding a medication to achieve some pressure reduction.
The iStent, for example, yields very little pressure reduction, only slightly better than cataract surgery alone. “It’s probably good for some group of patients who are highly selected,” Dr. Caprioli said. A patient with mild glaucoma who doesn’t need a low target pressure and is intolerant of medication might benefit from a MIGS procedure, he said. “MIGS may have a niche, but the niche is small.”
CONTRAINDICATIONS. Dr. Francis cited several contraindications for MIGS procedures.
- A very low pressure requirement.
- Patients with neovascular glaucoma. “The outflow system is scarred and nonfunctional, so we avoid angle-based surgeries in these patients,” he said. “There may also be blood vessels over the angle that will bleed during surgery.”
- Primary angle-closure patients, unless you can perform cataract surgery and goniosynechialysis at the same time. “Access to the angle outflow systems is poor, and the opening may scar if there is close iris proximity to the angle.”
- The presence of a too shallow anterior chamber, which increases the risk of damage to the corneal endothelium and/or iris. This also limits surgeon’s visibility.
WHERE ARE THE DATA? No published study has directly compared MIGS procedures against one another; moreover, we don’t yet have long-term data on success rates or evidence that MIGS slows visual field progression, Dr. Kaplowitz said.
“There’s really a need to have well-designed studies to evaluate the safety and efficacy of these procedures,” said Steven J. Gedde, MD, at Bascom Palmer Eye Institute in Miami. He noted that a number of studies have reported the outcomes of MIGS in conjunction with cataract surgery. “But you’re left wondering how much of the pressure reduction is due to MIGS and how much is due to cataract extraction.” However, one example of a well-designed study evaluating MIGS was cited by Dr. Gedde: a randomized clinical trial by Dr. Samuelson and his colleagues that compared cataract surgery alone to cataract surgery with an iStent.3
Any studies pertaining to MIGS devices will need to demonstrate safety and efficacy at least comparable to current Schlemm’s canal–based procedures, Dr. Samuelson said. He added that if the longer-term safety data prove as promising as the first couple years seem to indicate, and if efficacy improves even slightly, the treatment paradigm could shift for the roughly 75 percent of glaucoma patients who take one or two drops.
Ideally, as Dr. Samuelson said, “MIGS devices could make glaucoma more of a surgical disease and reduce the problems associated with eyedrops—compliance, expense, and side effects.”
Time will tell whether this proves to be the case.
__________________________
1 Francis BA, Winarko J. Dev Ophthalmol. 2012;50:125-136.
2 Kaplowitz K et al. Br J Ophthalmol. 2014;98(5):579-585.
3 Samuelson TW et al. Ophthalmology. 2011;118(3):459-467.
For Further Reading
- In February, the FDA Center for Devices and Radiological Health held a public workshop on MIGS.
- And in August, researchers published three year outcomes of a combined approach: endoscopic cyclophotocoagulation plus phacoemulsification. Francis BA et al. J Cataract Refract Surg. 2014;40(8):1313-1321.
|
Meet the Experts
JOSEPH CAPRIOLI, MD Professor of ophthalmology and chief of the Glaucoma Division at the Jules Stein Eye Institute, Los Angeles. Financial disclosure: None related to this topic.
BRIAN A. FRANCIS, MD, MS Professor of ophthalmology at the Doheny Eye Institute, Los Angeles. Financial disclosure: Consults for NeoMedix.
STEVEN J. GEDDE, MD Professor of ophthalmology and vice chair of education at the Bascom Palmer Eye Institute, Miami. Financial disclosure: None related to this topic.
KEVIN KAPLOWITZ, MD Assistant professor of ophthalmology at Stony Brook University, Stony Brook, N.Y. Financial disclosure: None.
THOMAS W. SAMUELSON, MD Attending surgeon, Minnesota Eye Consultants, and adjunct associate professor of ophthalmology, University of Minnesota, Minneapolis. Financial disclosure: Consults for AqueSys, Glaukos, Ivantis, and Transcend. |