By Lori Baker-Schena, MBA, EdD, Contributing Writer, interviewing Kaweh Mansouri, MD, MPH, Harry A. Quigley, MD, and Arthur J. Sit, SM, MD
Download PDF
Sixteen seconds a year. This is all the time clinicians generally take to measure intraocular pressure (IOP) in stable glaucoma patients using traditional tonometry, said Arthur J. Sit, SM, MD.
“It’s only a snapshot,” said Dr. Sit, at the Mayo Clinic in Rochester, Minn. “Even if we saw our glaucoma patients every day, we are still measuring the pressure for just a few seconds. Can you imagine if you only measured blood sugar levels in a diabetic every 3 months or if blood pressure could only be measured in a physician’s office? Without continuous measurement, it is difficult to obtain the big picture.”
Beyond the Snapshot
Clinical trials have shown an association between IOP variation and glaucoma progression,1,2 “yet we are clearly missing most of the IOP variations with our current technology,” Dr. Sit said. “There is definitely a need to measure IOP more frequently.”
This need to capture a more complete picture of a patient’s IOP has led to the development of extra- and intraocular 24-hour continuous IOP-monitoring devices.3
Contact lens sensor. One extraocular device is Sensimed’s contact lens sensor (CLS) Triggerfish, which received the CE mark in 2010 and FDA approval in March 2016. The Triggerfish is designed to monitor IOP fluctuations continuously for 24 hours in an ambulatory setting.
Implantable microsensor. Implandata Ophthalmic Products is currently conducting clinical research for its first product, Eyemate, an IOP microsensor that can be implanted in the ciliary sulcus during cataract or glaucoma surgery. The device has not yet received a CE mark, and some clinicians argue that it is not ready for clinical use (see “A Look to the Future”).
 |
|
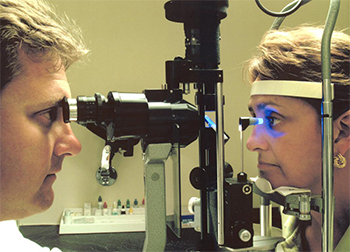
EVOLVING PRACTICE. While applanation tonometry remains the gold standard for measuring IOP, 24-hour monitoring may offer added value.
|
Triggerfish Update
How it works. The Triggerfish CLS consists of a highly oxygen-permeable soft contact lens with 2 sensing-resistive strain gauges that record circumferential changes in the area of the corneoscleral junction. The device does not actually measure IOP. Instead, it records fluctuations in the ocular dimensions, “which, it is assumed, correspond to changes in IOP, intraocular volume, and biomechanical properties of the eye,” said Kaweh Mansouri, MD, MPH, of the Montchoisi Clinic in Switzerland and the University of Colorado.
The device transmits data wirelessly from the sensor to an adhesive periorbital antenna that then sends the information via cable to a portable data recorder worn by the patient. Dr. Mansouri noted that 300 data points are acquired during a 30-second measurement period, repeated every 5 minutes. The data are then transferred via Bluetooth to the clinician’s computer.
Potential challenges. Dr. Mansouri noted that interpreting results from the device is challenging.
Processing the data. First, the clinician must process large amounts of data collected over 24 hours, which is in sharp contrast to the single measurement obtained when using Goldmann applanation tonometry (GAT). To help with this process, Dr. Mansouri’s group developed “an analytical approach using cosinor rhythmometry modeling to help interpret CLS data,”4 he said. “It is an objective method of assessing the circadian IOP rhythms, and it provides more information than we can obtain taking IOP measurements a few times a year.”
Measuring IOP. Second, the output signal does not directly measure IOP in millimeters of mercury (mm Hg). Instead, it measures IOP volume changes, using an arbitrary unit (a.u.) proportional to the electric signal (in mV) generated by the strain gauge embedded in the contact lens, Dr. Mansouri said.
“The absolute values in a.u. are not directly comparable among different eyes because of the internal recalibration process of the sensor,” he said. At present, there is no single calibration factor that allows conversion to mm Hg—and thus no ability to directly compare absolute pressure values from different sessions.
Potential benefits. The Triggerfish CLS is able to provide vital information about ocular biomechanics, Dr. Mansouri said. He cited a study in which IOP-related parameters obtained from a 24-hour recording of CLS measurements were associated with the rate of visual field progression in treated patients with glaucoma.5 The CLS parameters included the number of large peaks, mean peak ratio, wake-to-sleep slope, amplitude and area under the cosine curve, and variability from the mean. The authors stated, “A combination of CLS parameters obtained during a single 24-hour session provides a signature that seems to explain the rate of glaucoma progression better than a summary of office-hour IOP measurements in multiple visits.”5
Issues of cost. At present, the average cost is $650 for 1 Triggerfish CLS monitoring 1 eye for 24 hours. (This figure includes device-related costs plus the clinician’s time.) “The reimbursement is not adequate,” Dr. Mansouri said. “It is a matter of economies of scale. As we see the technology become widely adopted, I expect reimbursement to improve.”
If the CLS helps clinicians individualize treatment according to the patient’s fluctuation profiles, its cost may be offset by a decrease in the need to switch from one treatment to another, Dr. Mansouri said. “In addition, if we see IOP that is lower at night than during the day, for example, we can choose the timing of drug delivery according to the fluctuation patterns. These patterns may also provide guidance as to whether to choose laser or surgery for treatment-resistant patients.”
He added, “The faster we can pinpoint the right treatment for our glaucoma patients, the bigger the possibility of reducing the amount of disease progression, which also can save medical costs.”
Dr. Sit noted that if there is “strong evidence” that 24-hour monitoring provides valuable insight in a clinical setting, it will be worth the cost. “However,” he added, “if the 24-hour patterns in some patients are poorly reproducible, then multiple 24-hour monitoring sessions may be required, which increases the costs.”
More research needed. In any event, more research is needed before the data from the CLS device can be widely translated into clinical practice,6 Dr. Mansouri said. This includes clarifying the significance of these 24-hour patterns, the role of IOP-lowering medications on IOP patterns, and the impact of nocturnal IOP changes on glaucoma and progression. Prospective data are also needed to demonstrate how 24-hour monitoring can improve the long-term prognosis for glaucoma patients.
Research Update: Sleep and Limbal Strain
Harry A. Quigley, MD, at the Wilmer Eye Institute, said that the Triggerfish CLS proved valuable in his research to determine whether the eyes of patients with glaucoma may be damaged during sleep.
According to Dr. Quigley, aerospace engineer Alison B. Flatau, PhD, at the University of Maryland, was “interested in glaucoma and whether patients who sleep pressing their eye into a pillow increased limbal strain,” Dr. Quigley said. “For this research, we needed a device that could continually measure limbal strain during sleep produced by the mechanical force of the eye when it is in contact with a pillow.”
Dr. Quigley and his colleagues used the CLS, collecting data every 5 minutes during intervals of up to 60 minutes in various positions (sitting, lateral decubitus, supine, and face down—with the CLS-instrumented eye toward the pillow—during simulated sleep).
Using multivariable linear regression, the face-down position was associated with an increase in limbal strain in glaucomatous eyes but not in the control eyes. In addition, said Dr. Quigley, “those patients with glaucoma who had past visual field tests that had gotten worse had the biggest changes.”1
Dr. Quigley noted that his findings, in combination with those from C. Gustavo De Moraes, MD, of Columbia University in New York, indicate that fluctuations measured in 24-hour periods could be relevant in treating glaucoma. “Our next step is to study patients when they are actually sleeping, and use some type of protective eye shield to see if we can prevent sleep position–induced limbal strain.”
___________________________
1 Flatau A et al. JAMA Ophthalmol. 2016;134(4):375-382.
|
A Look to the Future
The Eyemate device, which is implanted in the ciliary sulcus, is a wireless intraocular transducer with 8 pressure and temperature sensors. The patient uses a handheld reader unit that receives data from the device and displays the IOP value.7 One-year results from the ARGOS study showed that all 6 patients maintained control of their glaucoma and were able to successfully perform self-tonometry at home.8 However, the device yielded inconsistent measurements over time. “There are definitely some growing pains” with the Eyemate device, Dr. Sit commented. “While the data indicate a good proof of concept, and there may be improvements beyond the published data, from what I have seen so far it is not yet ready for clinical use.”
Dr. Sit noted that while GAT remains the current gold standard, the ability to provide 24-hour patterns could be of added value. “Triggerfish is the first device of what I predict will be many devices introduced in the next few years, and future devices may provide true continuous IOP monitoring in addition to 24-hour IOP patterns.”
He added, “My hope is that 24-hour measurement becomes routine for glaucoma patients, with either the temporary extraocular noninvasive or intraocular invasive approaches. I see glaucoma residents in 2025 looking back and shaking their heads [over the fact] that we once depended on a snapshot of IOP rather than continual monitoring.”
___________________________
1 Lee PP et al. Am J Ophthalmol. 2007;144(6):901-907.
2 Hong S et al. Arch Ophthalmol. 2007;125(8):1010-1013.
3 Aptel F et al. Prog Retin Eye Res. 2016 Jul 28. doi:10.1016/jpreteyeres.2016.07.002.
4 Mansouri K et al. Invest Ophthalmol Vis Sci. 2012;53(13):8050-8056.
5 De Moraes CG et al. Ophthalmology. 2016;123(4):744-753.
6 Mansouri K. J Ophthalmic Vis Res. 2014;9(2):260-268.
7 Ittoop SM et al. Adv Ther. 2016;33(10):1679-1690.
8 Koutsonas A et al. Invest Ophthalmol Vis Sci. 2015;56(2):1063-1069.
___________________________
Dr. Mansouri is consultant ophthalmologist at the Montchoisi Clinic, Swiss Vision Network, in Lausanne, Switzerland, and adjoint associate professor of ophthalmology at the University of Colorado’s Anschutz Medical Campus in Aurora, Colo. Relevant financial disclosures: Sensimed: C, S.
Dr. Quigley is the A. Edward Maumenee Professor of Ophthalmology and director of the Glaucoma Center of Excellence at the Wilmer Eye Institute in Baltimore. Relevant financial disclosures: Sensimed: C.
Dr. Sit is associate professor of ophthalmology, glaucoma fellowship director, and vice chair for clinical practice in the ophthalmology department at the Mayo Clinic in Rochester, Minn. Relevant financial disclosures: AcuMEMS: C; InjectSense: C.
See the disclosure key at www.aao.org/eyenet/disclosures.